Proteins
Proteins consist of chains of 20 different kinds of amino acids connected by covalent linkages called peptide bonds. All amino acids have the same generalized structure as shown in Figure 2-3. An α-carbon is at the center of each amino acid. To its left (as conventionally written) is a basic (when ionized) amino group (NH3+). To the right of the a-carbon is an acidic (when ionized) carboxyl group (COO-). Ahydrogen atom forms a third bond to the a-carbon, and the fourth bond connects to a side-chain group (R).Amino acids are classified according to the nature of the R group. The 20 different amino acids used in the synthesis of proteins are symbolized by either three letter or single letter abbreviations as listed in Table 2.1.
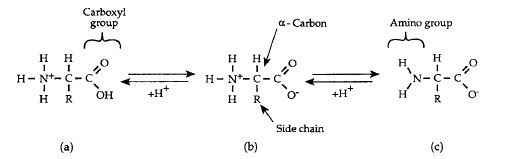 |
| Figure 2-3 Generalized structure of amino acids at different pH values. Predominant forms in (a) acidic, (b) neutral (pH 7), and (c) basic solutions. |
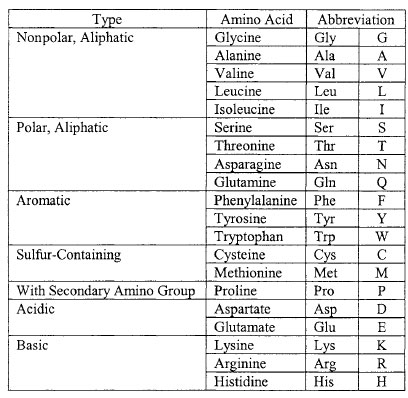 |
| Table 2.1 Amino acids grouped by chemical type. |
Polypeptides may differ by the number and kinds of individual amino acids they contain. The final structure can be described on four levels of increasing complexity. The primary structure of a functional protein consists of the linear sequence of amino acids in each of its polypeptide chains. There are two major kinds of secondary protein structure: α-helix and β-pleated sheet. An α-helix forms when a carbonyl (C=O) adjacent to one peptide bond is linked by a hydrogen bond to an amino group (NH) flanking a peptide bond in an amino acid about four residues along the same chain. β-pleated sheets form when hydrogen bonds form between amino acids on adjacent, parallel polypeptide strands. The polypeptide chain may fold back upon itself, forming weak, internal bonds (e.g., hydrogen bonds, ionic bonds) as well as stronger covalent disulfude bonds that stabilize its tertiary structure into a precisely and often intricately folded pattern. These bonds are formed from the side chains of different amino acid residues. If two or more polypeptide chains spontaneously associate, they form a quaternary structure.
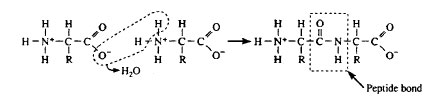 |
| Figure 2-4 Dehydration synthesis of a dipeptide by the formation of a peptide bond. |
Proteins perform many enzymatic, structural, and other roles in living systems. For example, they are a major structural component of ribosomes, they may act as hormones that signal between different cell types, or they may assist in the movement of organelles within the cell and movement of the cell itself.
Notes
An average polypeptide contains about 300 residues.
RNA has uracil in place of thymine.




