Translation in Prokaryotes
Ribosomes contain two sites for binding tRNA molecules: the aminoacyl site (A site), where each tRNA molecule first attaches, and the peptidyl site (P site), where a tRNAholds the growing polypeptide chain. The bacterial ribosome has two major subunits: a 30S subunit to which the mRNA and tRNAs become bound, and a 50S subunit to which tRNAs also bind. Each tRNA becomes charged by a tRNA aminoacyl synthetase that attaches a specific amino acid to each species of tRNA. Each tRNA molecule has a loop containing a triplet of ribonucleotides, called the anticodon, that can base pair with a complementary triplet codon in mRNA.Translation in prokaryotes begins at a start codon. The bacterial mRNA translation initation codon (AUG) encodes N-formylmethionine, whereas internal AUG codons specify methionine. The 3'-UAC-5' anticodon of the tRNApairs (in antiparallel fashion) with the complementary 5'-AUG-3' codon in the mRNA.
In the first step of translataion initiation, three protein initiation factors (IF1, IF2, IF3) and GTP bind to the 30S ribosomal subunit (Figure 5-1a). The 30S subunit binds to a Shine-Dalgarno (S-D) recognition sequence on the mRNA due to base pairing interactions with the 16S rRNA component of the ribosome. Then, a tRNA loaded with the amino acid N-formylmethionine (tRNAfMet) binds to the 30S-mRNA complex (Figure 5-1b). IF3 is released and then GTP hydrolysis releases IF1, IF2, GDP, and phosphate, allowing the 50S subunit to join the 30SmRNA- tRNAfMet complex to form a complete 70S initiation complex (Figure 5-1c,d). The tRNAfMet ends up in the P site of the ribosome. This completes the initiation phase.
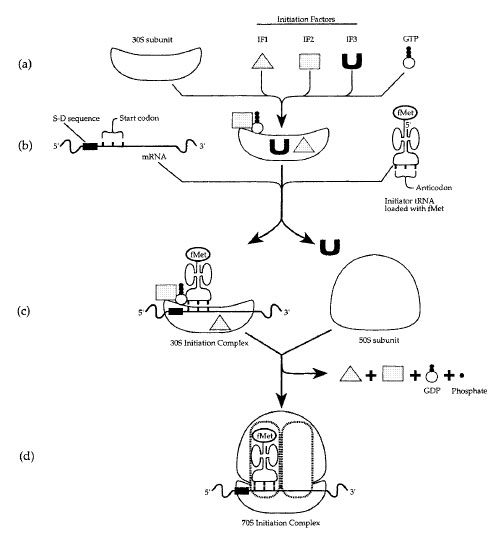 |
| Figure 5-1 Initiation of translation in bacteria. |
The elongation phase proceeds with the help of a group of protein elongation factors when a second activated tRNAenters the Asite (again by specific codon-anticodon base pairing). This places N-formylmethionine and the incoming amino acid next to one another so that a peptide bond can be formed between them by action of an enzymatic, ribosomal component called peptidyl transferase. The amino-acyl bond that held the N-formylmethionine to the 3' end of its tRNA is broken simultaneously with the formation of the peptide bond. The mRNA translocates three nucleotides through the ribosome and a new codon is now positioned in the A site. This process is repeated until a stop codon is encountered at the A site (Figure 5-2).
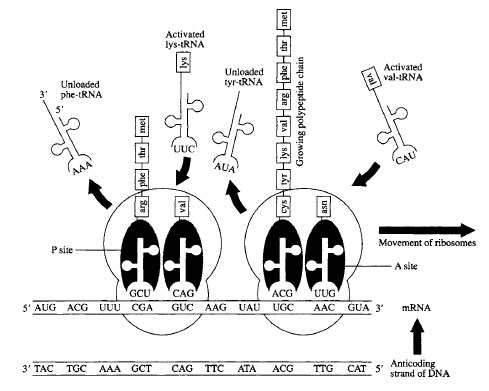 |
| Figure 5-2 Diagram of protein synthesis. |
At termination, the polypeptide is released from the tRNA with the help of release factors. The mRNA and the last tRNA are released from the ribosome as the ribosome dissociates into its 30S and 50S subunits.
Notes
Many antibiotics block protein synthesis by binding to prokaryotic ribosomal subunits.




