Selenium in the Soil
The two forms of selenium that predominate in cultivated soils are SeO42- and SeO32- (8). Soils also contain organic selenium compounds such as Se-Met (67). Selenium occurs in the highest concentration in the surface layers of soils, where there is an abundance of organic matter (9). Selenium in soils is generally considered to be controlled by an adsorption mechanism rather than by precipitation–dissolution reactions (68). In acid soils, sesquioxides control the sorption of selenium. Absorption controls the co-precipitation of SeO32- by Fe(OH)3. In mineral soils, SeO42- was absorbed by soil solids. Adsorption is also believed to control the distribution of selenium in the soil under oxidizing conditions (68).Transformation of SeO32- to SeO42- and vice versa occurs very slowly. The transformation of SeO32- to Se0 was found to be even slower (9). After Se0 is added to soil, it oxidizes rapidly to SeO32-. But, after the initial oxidation, the remaining selenium in the soil becomes inert, and any further oxidation proceeds very slowly. The rate of oxidation will vary in different soil types (68).
Geological Distribution
Selenium attracts interest because the amount in which it is present in soils is not evenly distributed geographically. Seleniferous soils and vegetation in North America extend from Alberta, Saskatchewan, and Manitoba south along the west coast into Mexico (12). The mean total selenium in soils of the United States is reported to be 0.26 mg kg-1 (69). Considerable variability exists from one location to another, and high Se concentrations occur in a few localized regions. In the United States, seleniferous soils occur in the northern Great Plains states of North Dakota, South Dakota, Wyoming, Montana, Nebraska, Kansas, and Colorado and in the Southwest states of Utah, Arizona, and New Mexico. These soils average 4 to 5 mg Se kg-1 and can reach levels as high as 80 mg kg-1 in some areas (8). The primary selenium sources are the western shales of the Cretaceous Age and the carbonic debris of sandstone ores of the Colorado Plateau (9).In the other parts of the world, selenium occurs in high amounts only in the semi-arid and arid regions derived from cretaceous soils (14). Seleniferous soils occur in Mexico, Columbia, Hawaii, and China. Toxic soil selenium levels (<300 mg kg-1) in Europe are limited to a few locations in Wales and Ireland (16). High-selenium soils also occur in Iceland, probably because of the volcanic activity on the island (16). In contrast, soils in Denmark, the Netherlands, Switzerland, Australia, and New Zealand, and Finland are naturally low in selenium (16). In humid climates, or in irrigated areas, most of the selenium is leached from soils (9). The most severe selenium-deficient area in the world is the Keshan region in southeastern China (16), where many children have died owing to insufficient dietary selenium. Variations in soil selenium can give rise to differences of selenium in the food chain (70).
Selenium can enter the soil through weathering of selenium-containing rocks, volcanic activity, phosphate fertilizers, and water movement. The selenium content in the soil reflects the concentration in the parent material, secondary deposition or redistribution of selenium in the soil profile, accumulation and deposition by selenium-accumulating plant materials, and erosion from selenium- containing rocks (71). The highest amounts of selenium are in igneous rock formations, existing as Se2- or sulfoselenides with copper, silver, lead, mercury, and nickel (8). Selenium also occurs under sedimentary rock formations. The weathering of selenium-containing rocks under alkaline and well-aerated conditions releases selenium into the soil, which oxidizes it into the SeO42- form. Selenium released from rocks under acidic, poorly aerated conditions will form insoluble Se2- and SeO32-. These forms of selenium develop stable adsorption complexes with ferric hydroxide and are less available to plants (8). The level of selenium in a phosphate fertilizer is governed by the concentration of selenium in the phosphatic rock (9). Fifteen different rock-phosphate fertilizers from sources in Canada and the United States ranged in selenium concentration from 0.07 to 178 mg kg-1 (72). Ordinary and concentrated super phosphate can be expected to contain between 40 and 60% more selenium than the phosphate rock from which it was made (72).
The distribution of selenium in the soil profile is determined by factors such as soil type, amount of organic matter, soil pH, and to some extent, leaching caused by rainfall. Organic matter helps to retain selenium in the surface horizon and has a greater SeO3-fixation capacity than clay minerals do (9,16). Soil pH, aeration, water levels, and oxidation-reduction conditions have an effect on the form of selenium in the soil and its availability to plants. Selenates are highly soluble in water and do not have stable adsorption complexes, thereby making them highly leachable (8).
Metal selenides occur in metal sulfide ores of iron, copper, and lead. Selenium occurs in small quantities in pyrite and in the minerals clausthalite (PbSe), naumannite ((Ag,Pb)Se), and tiemannite (HgSe). The similarity of the ionic radii of Se2- (0.191 nm) and S2- (0.184 nm) results in substitution of Se2- for S2-. Soil pH will affect the capacity of clays and ferric oxides to adsorb selenium (10). Selenite has a strong affinity for sorption, especially by iron oxides like geothite, amorphous iron hydroxide, and aluminum sesquioxides. Adsorption of SeO32- is also a function of soil-particle concentration and composition, SeO32- concentration, and the concentration of competing anions such as phosphate (10). Being stable in reducing environments, Se0 can be oxidized to SeO32- and to trace amounts of SeO42- by some microorganisms.
Selenium availability in Soils
Soil texture can affect selenium availability and uptake by plants. Because of the adsorption of SeO32- to clay fractions in the soil, plants grown on sandy soils take up twice as much selenium as those grown on loamy soils (10). Organic matter has the ability to draw selenium from the soil solution (10). In general, selenium concentrations in plants will increase as the level of soil selenium increases, but will decrease with the addition of SO42- (10). Extraction of selenium from soils is increased when SO42- is used in the leaching process (9). The presence of low-molecular- weight organic acids in the soil-root interface resulted in the loss of SeO32- sorption sites on aluminum hydroxides (73). A decrease in total selenium accumulation from soils supplied with sodium selenate (Na2SeO4) resulted under conditions of increasing levels of sodium (NaCl) and calcium (CaCl) salinity for canola (Brassica napus L.), kenaf (Hibiscus cannibinus L.), and tall fescue (74).The chemical form of selenium in the soil is determined mainly by soil pH and redox potential (Figure 18.2). In alkaline soils, selenium is in the available SeO42- form. When soil conditions become neutral to acidic, sparingly soluble ferric oxide-selenite complexes develop. Since sparingly soluble forms dominate at low pH, liming of the soil to raise the pH also has an effect by increasing the availability of selenium to plants (9). This response to addition of lime is probably caused by the reduced absorption to clays and iron oxides, resulting from increases in the soil pH (75). In the soil solution, the pH can change the speciation of selenium present. Below pH 4.5, soluble selenium speciation was 71% SeO42- and 8% SeO32-. When the pH was 7.0, the percentages were 51% for SeO42- and 23% for SeO32-. After 105 days, SeO42- accounted for 22% and SeO32- for 20% at pH 4.5, and were 12 and 22%, respectively, at pH 7.0 (76).
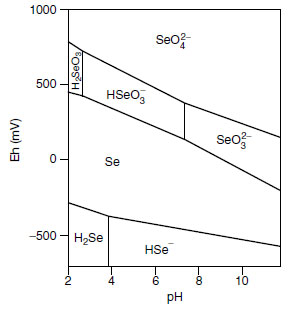 |
| FIGURE 18.2 Selenium speciation in an aqueous system: effect of pH and oxidation-reduction potential Eh. From R.L. Mikkelsen, et al., Selenium in Agriculture and the Environment. Madison, WI: American Society of Agronomy, Soil Science Society of America, 1989, pp. 65-94. |
Selenium can be supplied to plants by application to soil, by foliar sprays, and by seed treatments (16). Slow-release selenium fertilizers were effective over a 4-year period in maintaining selenium levels in subterranean clover (Trifolium subterraneum L.) to prevent selenium deficiency in sheep in Australia (77). Use of selenium-enriched Ca(NO3)2 significantly increased selenium in wheat (Triticum aestivum L.) (78). Coal fly ash has been used as a source of soil-applied selenium as well as many heavy metals (9). One should be careful when using phosphate fertilizers as soil amendments, since they may contain substantial amounts of selenium (10). Selenium incorporation into fertilizers is becoming common in some countries with low soil-Se levels. Spraying SeO42- onto pumice has been used for the production of selenium prills in New Zealand (16,77).




