History
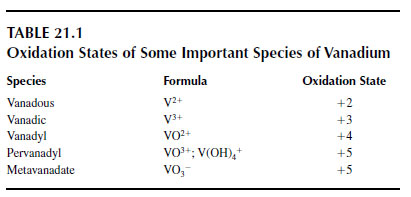
The transition element vanadium exists mostly in the +3, +4, and +5 oxidation states (Table 21.1), with the +4 and +5 states predominating under oxidizing conditions in the normal soil acidity of below pH 8 (1,2). Vanadium, with many other heavy metals, is released by anthropogenic activity, and its concentration has been steadily increasing in the environment. A study on peat dating back 12,370 years from a bog in Switzerland indicated a large increase in inputs of vanadium since the industrial revolution (3). Analysis of herbarium specimens of 24 species of vascular plants and 3 bryophytes collected over many years in Spain has shown a large increase in leaf vanadium concentrations, particularly since the 1960s (4).In soils, the main source of vanadium is from the burning of coal, and the subsequent addition of fly ash and bottom ash. In 1988, this ash contributed 11 to 67 x 106 kg V yr-1 to soils, 25% of the total vanadium deposited (5). Agricultural and food wastes contributed 3 to 22 x 106 kg yr-1, and atmospheric fallout added 3.2 to 21 x 106 kg yr-1.
 |
Total atmospheric fallout in a typical year in recent times (1983) resulted mainly from the burning of oil in electricity generation (estimated to be 6960 to 52,200-103 kg ) and from industrial and domestic combustion of oil (30,150 to 141,860-103 kg ) (5). Of the 15 heavy metals considered in that study, vanadium was the highest to be emitted during oil combustion (5), and its presence is often taken as an indicator of oil pollution (4).
In a study of microelements in the needles of white fir (Abies alba Mill.) in the Carpathian mountains of Eastern Europe, vanadium was found in high concentrations in the vicinity of ferrous metal plants (6), and it is emitted into the atmosphere during the production of copper, nickel, iron, and steel, and during the incineration of sewage sludge (5). With the discontinuation of sewage sludge incineration in many countries, it might be expected that direct addition of vanadium to soils in sewage sludge could increase worldwide.
The natural vanadium, occurring at approximately 110 to 150 mg kg-1 (1,7) in the crust of the Earth, is found particularly in roscoelite (KV3Si3O10(OH)2), vanadinite (Pb5(VO4)3Cl), and patronite (VS4) (1). During weathering of these rocks, vanadium is oxidized to the vanadate ion, which because of its solubility in water across a range of pH values makes vanadium readily available to plants. However, in practice, vanadium is not very mobile in soil, and in a study on a loamy sand, only a very small proportion of vanadium added to the top 7.5 cm of soil migrated down within 18 or 30 months; 81% remained in the top of the soil where it was added (2). The amount of vanadium that was removed by HCl-H2SO4 extraction of the top 7.5 cm of soil decreased by 81% during 18 months; hence, vanadium must have been transformed to an immobile form with time. Vanadium is known to adsorb to iron and aluminum oxides in the clay fraction (2). Some vanadium may be precipitated as Fe(VO3)2, and some may be immobilized by anion exchange (2).
The correlation is good between soil organic matter content and the oxidizable (immobile) fraction of vanadium (8). Insoluble humic acid is known to reduce mobile metavanadate (VO3-) anions to vanadyl (VO2+) cations, which probably bind to the humic acid by cation exchange (1). In an industrial area of Poland, most of the vanadium was bound to soil organic matter in a recent study of a soil that was rich in the element. The next largest fraction was the residual fraction followed, in order, by a fraction bound to iron-manganese oxides, a fraction in exchangeable form, and finally a fraction bound to carbonates in amounts too small to measure. The much lower amounts of vanadium in soil from an agricultural area occurred in the order of exchangeable fraction, residual fraction, the fraction bound to iron-manganese oxides, and the fraction bound to organic matter, with the fraction bound to carbonates being again too small to measure (9).
Uptake and accumulation are influenced by soil type, as soil composition affects the availability of vanadium. Vanadium generally is accumulated in plants in very small amounts in comparison to the total vanadium content of the soil (1). In a comparison of soybeans (Glycine max Merr.) grown in a fluvo-aquic soil and an Oxisol, an increase in shoot vanadium concentration occurred when concentrations of more than 30 mg V kg-1 were added to the fluvo-aquic soil, but no increase occurred at concentrations of up to 75 mg V kg-1 added to the Oxisol (10). Plant growth was inhibited when the concentration of vanadium supplied exceeded 30 mg kg-1 in the fluvo-aquic soil but was not inhibited in the Oxisol. In a study on bush bean (Phaseolus vulgaris L.), the accumulation of vanadium from a loamy sand was more than double the accumulation of cadmium and more than 300 times the accumulation of thallium (2). Concentrations of vanadium in plants are typically 0.27 to 4.2 mg kg-1 dry weight (11). At low rates of supply, vanadium appears to stimulate plant growth, but at higher rates of supply it appears to be toxic to many plants (7).




