Forms of Molybdenum in Soils
The speciation and availability of molybdenum in the soil solution is a function of pH. At water pH >5.0, molybdenum exists primarily as MoO42- (84), but at lower pH levels the HMoO4- and H2MoO40 forms dominate (44). For each unit increase in soil pH above pH 5.0, the soluble molybdenum concentration increases 100-fold (88). Plants preferentially absorb MoO42- and therefore the molybdenum nutrition of plants can be manipulated by altering soil acidity. Soil liming is commonly used to alleviate molybdenum deficiencies in plants by increasing the quantity of plant-available molybdenum in the soil solution (89), but the effect of liming on molybdenum nutrition varies by soil and plant type (Table 13.3). Excessive lime use may decrease the solubility of molybdenum through the formation of CaMoO4 (44), but Lindsay (90) suggests that this complex is too soluble to persist in soils. Using lime to change the acidity of a clay loam from pH 5 to 6.5 resulted in greater molybdenum accumulation in cauliflower, alfalfa (Medicago sativa L.), and bromegrass (Bromus inermis Leyss.), but molybdenum accumulation was relatively unaffected if plants were grown in a sandy loam (Table 13.3) (87). For plants grown in sandy loam, lime and molybdenum were both required to significantly increase the molybdenum content of the plant tissue.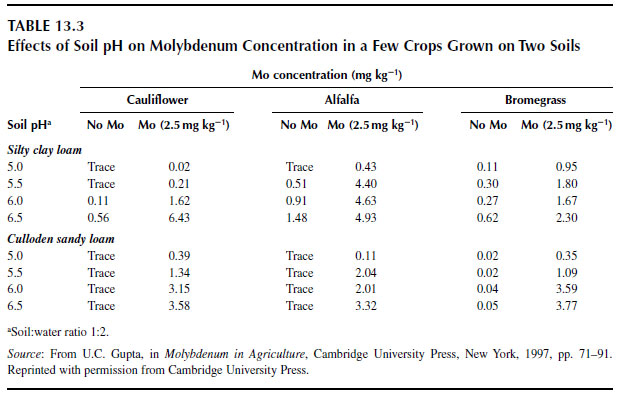 |
Significant amounts of molybdenum can be bound, or fixed, in soils by iron and aluminum oxides, particularly under acidic conditions (19). These sesquioxides have a pH-dependent surface charge that becomes more electrically positive as soil pH decreases, and more negative as soil pH increases. Changes in the surface charge are due to the protonation and deprotonation of surface functional groups (91). Under acidic soil conditions, the molybdate anion is adsorbed strongly to the surface of iron and aluminum oxides by a ligand exchange mechanism (92), and adsorption is greatest at pH 4 (83). In acid soils the molybdenum concentration in the soil solution can be reduced greatly, but because molybdenum is adsorbed weakly to soils and hydrous oxides at alkaline pH, these soils have a relatively large proportion of molybdenum in the solution phase (93). Compared with adsorption on hydrous iron oxides, the strength of molybdenum adsorption to aluminum oxide is much weaker (94). Despite this difference, aluminum oxides play an important role in the sorption of molybdenum in soils. For instance, the adsorption capacity of montmorillonite increases in the presence of interlayered aluminum hydroxide polymers (85).
Molybdenum also exists in soils as a constituent of various molybdenum-containing minerals. The primary source of molybdenum in soils is molybdenite (MoS2), but other minerals also contribute to the molybdenum content of soils, such as powellite (CaMoO4), wulfenite (PbMoO4), and ferrimolybdite (Fe2(MoO4)3 � 8H2O) (95). Of these minerals, only molybdenite and ferrimolybdite are mined commercially (83). In water-saturated soils, the availability of molybdenum is influenced by its reaction with other redox-active elements such as sulfur. Under strongly reducing conditions molybdenum forms sparingly soluble thiomolybdate complexes, with MoS2 being the most important mineral controlling molybdenum solubility (44). Other minerals whose ions are also affected by oxidation-reduction state, such as MnMoO4 or FeMoO4, are too soluble to precipitate in soils (92). Soil pH greatly influences the availability of molybdenum from these mineral sources; even PbMoO4, the least soluble of the possible soil compounds, becomes more soluble as pH increases (87).
Soil organic matter has been found to complex or fix molybdenum in soils, but the mechanisms of sorption are not well understood. Molybdenum binds strongly to humic and fulvic acids (92). Owing to the great affinity of molybdenum to be fixed by organic matter, its concentration in forest litter can reach 50 mg Mo kg-1 (44). The accumulation of molybdenum in organic matter can be particularly high if soil drainage is impeded (95). Organic-matter-rich soils can supply adequate amounts of molybdenum for plant growth due to a slow release of molybdenum from the organic complex (44). However, there are conflicting reports concerning the effect of soil organic matter on the availability of molybdenum in the soil solution. Plant-available molybdenum has been reported to be low in soils having high quantities of organic matter (96), particularly on peat soils due to the strong fixation of molybdenum by humic acid (44). In contrast, Srivastiva and Gupta (85) suggested that soil organic matter increases the available molybdenum content of acid soils by inhibiting the fixation of MoO42- by sesquioxides.




