Foliar Absorption
Tank mixing urea-ammonium nitrate fertilizer (UAN; 0.5% by weight) with ZnSO4 increased leaflet zinc concentration compared to using ZnSO4 alone in pecan. Zinc nitrate was more efficient than ZnSO4 in increasing leaflet concentration, especially if tank mixed with UAN (0.5%). Zinc concentrations of spray solutions can be reduced by one eighth to one fourth of the current recommended rate as ZnSO4 at 86 g per 100 L of water. Use of the lowest rate of Zn(NO3)2, 10.8 g per 100 L of water + UAN, increased yield and income over the recommended rate of ZnSO4 (66). This paper plus earlier work that led to the formulation of Zn(NO3)2 + UAN was patented under the name NZN. (NZN® was patented in 1971 by J. Benton Storey and Allied Chemical Co. under the trade mark registration No. 1041108). The work was documented by Storey and coworkers (34,45,46,66–75).Grauke (76) followed with research which evaluated and expanded previous work with NZN and considered problems of precipitation of zinc in spray formulations. He noted that precipitation of ZnSO4 occurs from NZN stock solutions with 5% Zn and that use of solutions with 1% Zn avoided precipitation. Earlier,Wallace et al. (77) reported increasing absorption of zinc from ZnSO4 with increasing alkalinity up to pH 8. However, use of high-pH zinc formulations is limited because of low stability of the formulations and the precipitation of zinc when stock solutions of high pH are diluted with water. To avoid precipitation, the ZnSO4 and UAN should be sprinkled into an agitated, full tank of water (76).
Pecan and corn (Zea mays L.) leaves absorbed more Zn from NZN than from ZnSO4 , and absorption of both formulations was increased at high humidity. Grauke (76) noted that differences in the absorption of the formulations were related to their effective concentrations, calculated by multiplying the molecular concentration of the solution by its activity coefficient. Activity coefficients are factors which, when multiplied by the molar concentrations, yield the active mass or effective concentration. Activity coefficients may be calculated for solutions are less that 0.01 M by using the Debye-Huckel equation
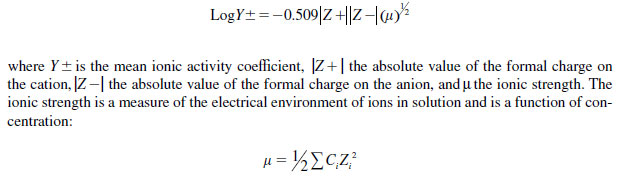 |
where Σ is the sum of the concentrations, Ci, for each ionic species multiplied by the formal charge Zi on the ith ion. For example, a 200 mg L-1 solution of Zn(NO3)2 has an ionic strength (�) of 0.009. When that figure is used in the above equation, the activity coefficient (Y±) is equal to 0.597. When each of these factors are multiplied by the mole concentration of the solutions, which is 0.003 for each solution, the active mass of respective solutions is obtained: 0.0024 M (156.9 mg L-1) for Zn(NO3)2 and 0.0018 M (117.7 mg L-1) for ZnSO4. Therefore, although equal concentrations of the two solutions were applied, the active mass of the ZnSO4 solution was only 75% of that in the Zn(NO3)2 solution.
Application of a 10-�L drop of a 200 mg L-1 solution of 65ZnSO4 resulted in sorption of 46% of the applied label. The portion of the applied label absorbed by a leaf treated with a 10-�L drop of 200 mg L-1 65Zn(NO3)2 was 74%. Therefore, sorption from the ZnSO4 solution was 62% of that for the Zn(NO3)2 solution (76).
The inclusion of NH4NO3 and urea to either Zn(NO3)2 or ZnSO4 resulted in a significant increase in translocation of absorbed zinc. There was no significant difference in movement of absorbed zinc between ZnSO4 + NH4NO3 + urea and Zn(NO3)2 + NH4NO3 + urea. However, the total amount of zinc available to leaves treated with Zn(NO3)2 + NH4NO3 + urea would be greater, since much more of the applied zinc was absorbed. These data indicate that the efficiency of a foliar zinc application could be increased by using the Zn(NO3)2 + NH4NO3+urea treatment, which increases the amount of total zinc absorbed by the leaf as well as the percentage of absorbed zinc translocated from the treatment site. The latter two ingredients of the triad are contained in a commercial 32% N, liquid UAN fertilizer. Grauke's (76) meticulous evaluation of this triad proved that the presence of NH4NO3 + urea did not result in increased sorption of either Zn(NO3)2 or ZnSO4 as would be expected if urea facilitated cuticular penetration (78). Wadsworth (37) and Grauke (76) showed that Zn(NO3)2 increased zinc absorption more than ZnSO4 with or without urea. By increasing the total absorption of labeled zinc from Zn(NO3)2 and by increasing the translocation of absorbed zinc from NH4NO3 + urea, these treatment showed increased efficiency for foliar zinc fertilization.
A 1975 article in California Farmer (California Farmer was a trade journal that featured new products but was not given a publication number) reported positive response with NZN on almonds, cherries, peaches, apples, walnuts, grapes, tomatoes, and head lettuce. The NZN provides the leaf with zinc that is available for synthesis of IAA, which stimulates shoot growth and leaf expansion. The necessity of applying zinc when the cuticles are less formidable dictates application when the leaves are first developing. Most leaf expansion of bearing pecan shoots occurs in the first 2 months of growth, so zinc foliar sprays should be applied at first sign of the green tip emerging through the terminal bud scales. Subsequent foliar Zn sprays should be applied 1, 3, 5, and 8 weeks after green tip (74,79). These early season Zn sprays were based on the work by Wadsworth (37) with pecans and are also supported by the conclusion of Franke (80) that immature leaves with thinner cuticles were more absorptive than mature leaves and that the lower leaf surfaces, which also had thinner cuticles, were slightly more absorptive than the upper leaf surfaces. Labelled 65Zn absorbed by the immature leaves moved primarily acropetally and was deposited in the midrib and lateral veins of the treated leaf.
Small amounts of 65Zn were transported basipetally within the leaf from the treatment spot down the petiole into the transport system of the stem. Acropetal movement of 65Zn was consistently dramatic when 73 �g of Zn as ZnSO4, which contained 3.4 �Ci 65Zn, was applied to the stem of pecan seedlings by insertion under a phloem patch, thus proving that once zinc negotiates the cuticle there is no problem of rapid acropetal transport (37).
An important unique feature of NZN is its ability to transport zinc absorbed from a 10 �L droplet of 200 mg Zn L-1 labeled with 0.3 �Ci 65Zn. The percentage of absorbed zinc detected away from the treatment site was greater in leaves treated with NZN (81).
Landscape maintenance firms in the Southwest have long had problems with ZnSO4-induced defoliation of woody ornamentals and fruit trees during spraying of the large ubiquitous pecan trees in landscapes because of drift to landscape species that are susceptible to ZnSO4-induced defoliation. Foliar treatment of 18 species of container-grown woody ornamentals with NZN resulted in no spray damage (82). Zinc concentrations were increased in 13 species compared to untreated plants. Quality was improved in three species without a related increase in zinc content. The ornamentals in this study were not expected to benefit from zinc because they were growing in acid media.
Peach trees are notoriously susceptible to ZnSO4-induced defoliation (83). However, trees suffering from zinc deficiency may develop 'little leaf' if not supplied with zinc. In early practices, use of ZnSO4 was recommended commonly for control of bacterial leaf spot (Phytomonas pruni) (84,85). ZnSO4 was considered effective in controlling bacterial leaf spot on peaches in the 1940s, but the spray solution had to include hydrated lime to prevent defoliation (79). Storey Orchards was established on upland sand in 1932 in Red River county, Texas, and grew to 70 acres in the early 1940s. All of the labor, with the exception of harvest, was supplied by the three family members. My remembrance of childhood was spraying the 'Burbank July Elberta' trees with ZnSO4 for the control of bacterial leaf spot and use of hydrated lime to prevent ZnSO4 spray burn. Similarly, Sherbakoff and Andes (86) and Kadow and Anderson (84,85) reported that hydrated lime was used with lead arsenate (PbHASO4 ) to prevent leaf burn. Lead arsenate was used for plum curculio control (85). It is interesting to note that PbHASO4, ZnSO4, and Ca(OH)2 were last reported in a peach spray guide (87) in which DDT was mentioned first. DDT was far more effective in plum curculio control than PbHASO4, but its use diminished the amounts of zinc applied. Johnson et al. (88) published a spray guide that recommended a copper fungicide and eliminated the need for ZnSO4 in pest control. This recommendation also overlooked the value of ZnSO4 to supply zinc for tree vigor. Today, NZN is used to supply zinc without the danger of spray burn.
Some sandy soils where peaches are grown, such as in Hidalgo county in South Texas and the ridge in Florida (89), are zinc-deficient. In both areas the typical symptom of 'little leaf' was common. Arce (19,20) used three different zinc fertilizers in a Hidalgo county peach orchard. All three fertilizers gave excellent response in preventing little leaf.




