Plant Lab Protocols / Methodology for Nitrogen Fixation
Nitrogenase
Legume plants assume a special status among cultivated crops because of their symbiotic association with Rhizobia. The nodules formed by Rhizobium is a good example of coexistence in nature. The plant supplies photosynthates for the bacterium and in turn, the bacterium gives assimilated nitrogen to the plant. In these days of energy crisis, increasing the capacity of biological nitrogen fixation, is felt as an additional, if not an alternative to chemical fertilizers. Screening of legume root nodules for nitrogenase, the enzyme which converts nitrogen into ammonia, is commonly done in breeding programmes to identify improved legume plant type, and to identify efficient bacterial strain in agricultural microbiology. Nitrogenase has the capacity to reduce certain other compounds apart from its natural substrate, dinitrogen. Reduction of acetylene by nitrogenase is very well exploited for assaying nitrogenase.
Principle
Acetylene is reduced to ethylene by nitrogenase. The ethylene produced is measured in a gas chromatograph (GLC) and the activity is expressed as 'n' mole ethylene produced for unit time per g dry nodules.
Materials
» Gas Chromatograph with Flame Ionization Detector (FID)
» Air-tight Syringes
» Conical Flasks (100mL) with small mouth to fit Serum Caps
» Acetylene Gas
» Ethylene Gas
» GLC Operating Conditions
Carrier Gas - Nitrogen/Helium/Argon with a Flow Rate 30 to 45mL
Gas for Detector - Hydrogen and Air
Column - Poropak N, R, T and Q or Silica Gel
|
Poropak
|
Silica Gel
|
Oven/column temperature
|
60°C
|
150°C
|
Injector temperature
|
65°C
|
160°C
|
Detector temperature
|
85°C
|
175°C
|
Retention time for ethylene (min)
|
1.3
|
1.5
|
Retention time for acetylene (min)
|
1.8
|
3.0
|
Procedure
1.
|
Remove the plants from the soil without disturbing the root nodules.
|
2.
|
Excise the roots with nodules.
|
3.
|
Place the root system into a 100mL conical flask.
|
4.
|
Seal the flask with rubber septum (serum cap).
|
5.
|
Remove 10mL of air from the flask with an air tight syringe.
|
6.
|
Inject 10mL acetylene into the flask.
|
7.
|
Incubate for 30 to 60 min at room temperature.
|
8.
|
Remove 0.5 to 1mL gas mixture from the flask with an air-tight syringe.
|
9.
|
Inject the gas mixture into a pre-conditioned GLC.
|
10.
|
Look for the acetylene and ethylene peaks and measure the ethylene peak height.
|
11.
|
At the end of the experiment, detach the nodules and take the dry weight.
|
12.
|
For standard, inject 10mg(Z) of pure ethylene into a 100mL sealed conical flask to satisfy identical assay conditions. Remove 0.5 to 1mL (same volume as of the sample) and inject into the GLC and measure ethylene peak height.
|
Calculation
1.
|
Standard amount of ethylene (E) in mmol =
|
0.0446 x Z mL
|
Peak height in mm x attenuation
|
|
2.
|
Amount of ethylene produced in mmol in the sample
= E x Peak height of sample ethylene in mm x attenuation
|
3.
|
Activity of nitrogenase
= n mol or mmol ethylene per unit sample per unit time
(unit sample may be g dry weight nodules or mg dry weight cell or mg protein)
|
Notes
1.
|
Detaching nodules from the roots will give low nitrogenase activity. There should be no moisture at the roots.
|
2.
|
To minimize residual acetylene peak, 1mL of air in the flask may be removed and 1mL of acetylene injected instead of 10mL.
|
3.
|
If any subnormal acetylene peak is noticed, discard the sample. This may be due to leakage in the septum of the flask.
|
4.
|
If no peak is seen, check the needle for blockage. Clogging of needles often happens when very sharp needles are used.
|
5.
|
The septum in the injecting point of GLC should be changed for every 25 injections.
|
6.
|
Pure acetylene should be injected into GLC as blank to check for any contamination with ethylene. If any ethylene peak is noticed in acetylene blank, this peak height multiplied by the attenuation should be deducted from the sample ethylene peak height and attenuation.
|
7.
|
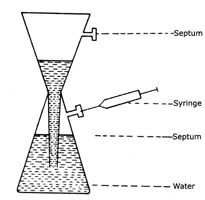
Generation of Acetylene
Acetylene may be generated from calcium carbide (CaC2) using the following glass apparatus (refer Figure 1)
- Completely fill lower reservoir with distilled water.
- Drop in two CaC2 pellets. To purge water of some dissolved air, wait for a few seconds before stoppering with serum cap.
- Withdraw portion of C2H2 using a syringe via serum cap.
- Periodically clean vessel with HC1 to remove Ca(OH)2
|
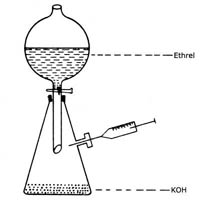
8.
|
Generation of Ethylene
Ethylene (99% pure) can be easily produced and samples withdrawn as shown in the Figure 2.
- Take 60mL 5M KOH in the round bottom flask.
- Take 100mL of ethrel (2 chloroethyl phosphonic acid - 40%) in the separating funnel.
- Add ethrel into the bottom flask.
- Allow the air to escape for a few min and then stopper the side arm outlet with a rubber septum.
- Remove ethylene gas whenever necessary with an air-tight syringe.
|
| |
 |
|
 |
|
| |
Fig. 1. Generation of Acetylene |
|
Fig.2. Generation of Ethylene |
|
References
1. Turner, G L and Gibson, A H (1980) In: Methods for Evaluating Biological Nitrogen Fixation (Ed Bergersen, F J) John Wiley and Sons NY pill.
|
Support our developers

More in this section