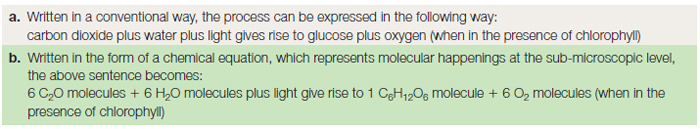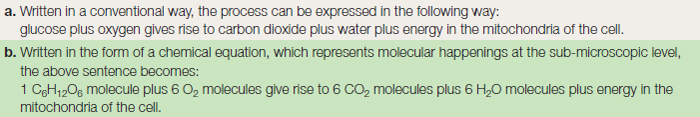Photosynthesis
Content
The following environmental requirements for photosynthesis are explained in detail below:
All the complex organic compounds, based on carbon, must be produced from the simple raw materials, water and carbon dioxide. Many organisms are unable to manufacture their own food, and must therefore feed on already manufactured organic matter such as plants or animals. Since large animals predate on smaller animals, which themselves feed on plants, all organisms depend directly or indirectly on photosynthesis occurring in the plant as the basis of a food web or chain. A summary of the process of photosynthesis is given in Table 8.1 as a word formula and as a chemical equation. This apparently simple equation represents, in reality, two different stages in the production of glucose. The first, the ‘light reaction’ occurs during daylight, and splits water into hydrogen and oxygen. The second, the ‘dark reaction’ occurring at night, takes the hydrogen and joins it to carbon dioxide to make glucose.
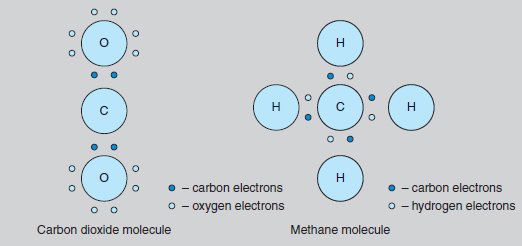
Most plant species follow a ‘C–3’ process of photosynthesis where the intermediate chemical compound contains three carbon atoms (C – 3) before producing the six-carbon glucose molecule (C – 6). Many C – 3 plants are not able to increase their rate of photosynthesis under very high light levels. In contrast, a ‘C– 4’ process is seen in many tropical families, including the maize family, where plants which use an intermediate compound containing four carbon atoms (C4) are able to continue to respond to very high levels of light, thus increasing their productivity. A third process, called ‘CAM’ (Crassulacean Acid Metabolism) was first discovered in the stonecrop family. Here, the intermediate chemical is a different four-carbon compound, malic acid. This third process has more recently been found in several other succulent plant families (including cacti), all of which need to survive conditions of drought. Such plants need to keep their stomata closed during the heat of the day, but this prevents the entry of carbon dioxide. During the night, carbon dioxide is absorbed and stored as malic acid, ready for conversion to glucose the next day. CAM plants do not normally grow very fast because they are not able to store large quantities of this malic acid, and thus their potential for glucose production is limited. Carbon chemistry All living organisms, from viruses to whales, have the element carbon at the centre of their chemistry. The study of this element’s chemical activity is called organic chemistry. Originally, it was thought that all organic compounds came from living organisms, but modern chemistry has brought synthetic urea fertilizer and DDT, which are organic. Unusually in the range of chemical elements, carbon (like silicon) has a combining power, or valency, of four. This means that each carbon atom can react with four other atoms, whether they are atoms of other elements such as hydrogen, or with more carbon atoms. Since the four chemical bonds from the carbon atom point in diametrically opposite directions, it can be seen that molecules containing carbon are three dimensional, a feature which is very important in the chemistry of living things. Carbon normally forms covalent (non-ionic) bonds in its molecules. Here, the bond shares the electrons, and so there is no chemical charge associated with the molecule (see also ionic bonds). Two of the simplest molecules to contain carbon are carbon dioxide and methane (see Figure 8.2). Carbon dioxide, or CO2 , is a constituent of the air we breathe and the sole source of carbon for almost all plants. In this compound, the carbon attaches to two oxygen atoms, each with a valency of two.
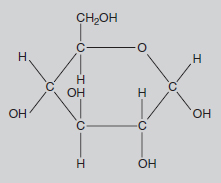
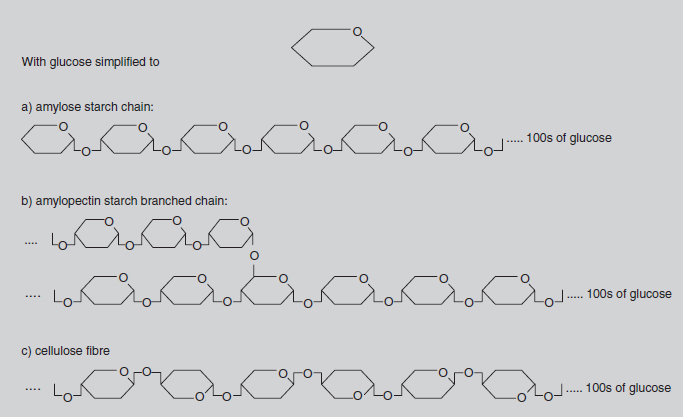
Methane, or CH4, is often referred to as ‘marsh gas’ and is the major constituent of the North Sea gas supply. In this compound, the carbon atom is bonded with four hydrogen atoms, each of which has a valency of one. Most fuels, such as petrol, are chemically related to methane, but with more carbon atoms in the molecule. This family of chemicals (containing progressively more carbons in the molecule are methane, butane, propane and octane) is collectively called the hydrocarbons. The molecules in petrol mainly contain eight carbon atoms, hence the term ‘octane’. Slightly more complicated is the glucose molecule (C6H12O6). Here there is a circular molecule and Figure 8.3 indicates its three-dimensional structure. Glucose is the molecule produced by photosynthesis. It is the starting point for the synthesis of all the many molecules used by the plant, i.e. starch, cellulose (see Figure 8.4), proteins, pigments, auxins, DNA, etc. These molecules may contain chains with hundreds of carbon atoms joined in slightly different ways, but the basic chemistry is the same. All the valency requirements of carbon, oxygen and hydrogen are still met.
Requirements for photosynthesis Carbon dioxide In order that a plant may build up organic compounds such as sugars, it must have a supply of carbon which is readily available. Carbon dioxide is present in the air in concentrations of 330 ppm (parts per million) or 0.03 per cent, and can diffuse into the leaf through the stomata. Carbon dioxide gas moves ten thousand times faster in air than it would in solution through the roots. The amount of carbon dioxide in the air immediately surrounding the plant can fall when planting is very dense, or when plants have been photosynthesizing rapidly, especially in an unventilated greenhouse. This reduction will slow down the rate of photosynthesis, but a grower may supply additional carbon dioxide inside a greenhouse or polythene tunnel to enrich the atmosphere up to about three times the normal concentration, or an optimum of 1000 ppm (0.1 per cent) in lettuce. Such practices will produce a corresponding increase in growth, provided other factors are available to the plant. If any one of these is in short supply, then the process will be slowed down. This principle, called the law of limiting factors, states that the factor in least supply will limit the rate of the process, and applies to other non- photosynthetic processes in the plant. It would be wasteful, therefore, to increase the carbon dioxide concentration artificially, e.g. by burning propane gas, or releasing pure carbon dioxide gas, if other factors were not proportionally increased.
Light Light is a factor required for photosynthesis to occur. In any series of chemical reactions where one substance combines with another to form a larger compound, energy is needed to fuel the reactions. Energy for photosynthesis is provided by light from the sun or from artificial lamps. As with carbon dioxide, the amount of light energy present is important in determining the rate of photosynthesis – simply, the more light or greater illuminance (intensity) absorbed by the plant, the more photosynthesis can take place. Light energy is measured in joules/square metre, but for practical purposes the light for plant growth is measured according to the light falling on a given area, that is lumens per square metre (lux). More recently, the unit ‘ microwatts per sq. metre’ has been introduced. One lux, in natural sunlight, is equal to microwatts per sq. metre. Whilst the measurement of illuminance is a very useful tool for the grower, it is difficult to state the plant’s precise requirements, as variation occurs with species, age, temperature, carbon dioxide levels, nutrient supply and health of the plant. However, it is possible to suggest approximate limits within which photosynthesis will take place; a minimum intensity of about 500–1000 lux enables the plant’s photosynthesis rate to keep pace with respiration, and thus maintain itself. The maximum amount of light many plants can usefully absorb is approximately 30 000 lux, while good growth in many plants will occur at 10 000–15 000 lux. Plant species adapted to shade conditions, however, e.g. Ficus benjamina , require only 1000 lux. Other shade-tolerant plants include Taxus spp., Mahonia and Hedera. In summer, light intensity can reach 50 000–90 000 lux and is therefore not limiting, but in winter months, between November and February, the low natural light intensity of about 3000–8000 lux is the limiting factor for plants actively growing in a heated greenhouse or polythene tunnel. Care must be taken to maintain clean glass or polythene, and to avoid condensation that restricts light transmission. Intensity can be increased by using artificial lighting, which can also extend the length of day, which is short during the winter, by supplementary lighting . This method is used for plants such as lettuce, bedding plants and brassica seedlings. Total replacement lighting Growing rooms which receive no natural sunlight at all use controlled temperatures, humidities, and carbon dioxide levels, as well as light. Young plants which can be grown in a relatively small area, and which are capable of responding well to good growing conditions in terms of growth rate, are often raised in a growing room. The type of lamp Lamps are chosen for increasing intensity, and therefore more photosynthesis. All such lamps must have a relatively high efficiency of conversion of electricity to light, and only gas discharge lamps are able to do this. Light is produced when an electric arc is formed across the gas filament enclosed under pressure inside an inner tube. Light, like other forms of energy, e.g. heat, X-rays and radio waves, travels in the form of waves, and the distance between one wave peak and the next is termed the wavelength. Light wavelengths are measured in nanometres (nm); 1 nm= one thousandth of a micrometre. Visible light wavelengths vary from 800 nm (red light – in the long wavelength area) to 350 nm (blue light – in the short wavelength area), and a combination of different wavelengths (colours) appears as white light. Each type of lamp produces a characteristic wavelength range and, just as different coloured substances absorb and reflect varying colours of light, so a plant absorbs and reflects specific wavelengths of light.
Since the photosynthetic green pigment chlorophyll absorbs mainly red and blue light and reflects more of the yellow and green part of the spectrum, it is important that the lamps used produce a balanced wavelength spectrum to include as high a proportion of those colours as possible, in order that the plant makes most efficient use of the light provided. The gas included in a lamp determines its light characteristics. The two most commonly used gases for horticultural lighting are mercury vapour, producing a green blue light with no red, and sodium, producing yellow light. This limited spectrum may be modified by the inclusion of fluorescent materials in the inner tube, which allow the tube to re-emit wavelengths more useful to the plant emitted by the gas and re-emit the energy as a shorter wavelength. Thus, modified mercury lamps produce the desirable red light missing from the basic emission. Low-pressure mercury-filled tubes produce diffuse light and, when suitably grouped in banks, provide uniform light close to plants. These are especially useful in a growing room, provided that they produce a broad spectrum of light as is seen in the ‘ full spectrum fluorescent tubes ’ . Gas enclosed at high pressure in a second inner tube produces a small, high intensity source of light. These small lamps do not greatly obstruct natural light entering a greenhouse and, while producing valuable uniform supplementary illumination at a distance, cause no leaf scorch. Probably the most useful lamp for supplementary lighting in a greenhouse is a high-pressure sodium lamp, which produces a high intensity of light, and is relatively efficient (27 per cent). Carbon dioxide enrichment should be matched to artificial lighting in order to produce the greatest growth rate and most efficient use of both factors. Temperature The complex chemical reactions which occur during the formation of carbohydrates from water and carbon dioxide require the presence of chemicals called enzymes to accelerate the rate of reactions. Without these enzymes, little chemical activity would occur. Enzyme activity in living things increases with temperature from 0°C to 36°C, and ceases at 40°C. This pattern is mirrored by the effect of air temperature on the rate of photosynthesis. But here, the optimum temperature varies with plant species from 25°C to 36°C as optimum. It should be borne in mind that at very low light levels, the increase in photo-synthetic rate with increased temperature is only limited. This means that any input of heating into the growing situation during cold weather will be largely wasted if the light levels are low. Integrated environmental control in a greenhouse is a form of computerized system developed to maintain near-optimum levels of the main environmental factors (light, temperature and carbon dioxide) necessary for plant growth. It achieves this by frequent monitoring of the greenhouse using carefully positioned sensors. Such a system is able to avoid the low temperature/light interaction described above. The beneficial effects to plant growth of lower night temperatures compared with day are well known in many species, e.g. tomato. The explanation is inconclusive, but the accumulation of sugars during the night appears to be greater, suggesting a relationship between photosynthesis and respiration rates. Such responses are shown to be related to temperature regimes experienced in the areas of origin of the species.
Adaptation to extremes in temperature can be found in a number of species; for example resistance to high temperatures above 40°C in thermophiles ; resistance to chilling injury is brought about by lowering the freezing point of cell constituents. Both depend on the stage of development of the plant, e.g. a seed is relatively resistant, but the hypocotyl of a young seedling is particularly vulnerable. Resistance to chilling injury is imparted by the cell membrane, which can also allow the accumulation of substances to prevent freezing of the cell contents. Hardening off of plants by gradual exposure to cold temperatures can develop a change in the cell membrane, as in bedding plants and peas. Examples of plant hardiness are found in Table 4.3. Water Water is required in the photosynthesis reaction but this represents only a very small proportion of the total water taken up by the plant (see transpiration). Water supply through the xylem is essential to maintain leaf turgidity and retain fully open stomata for carbon dioxide movement into the leaf. In a situation where a leaf contains only 90 per cent of its optimum water content, stomatal closure will prevent carbon dioxide entry to such an extent that there may be as much as 50 per cent reduction in photosynthesis. A visibly wilting plant will not be photosynthesizing at all. Minerals Minerals are required by the leaf to produce the chlorophyll pigment that absorbs most of the light energy for photosynthesis. Production of chlorophyll must be continuous, since it loses its efficiency quickly. A plant deficient in iron, or magnesium especially, turns yellow (chlorotic) and loses much of its photosynthetic ability. Variegation similarly results in a slower growth rate.
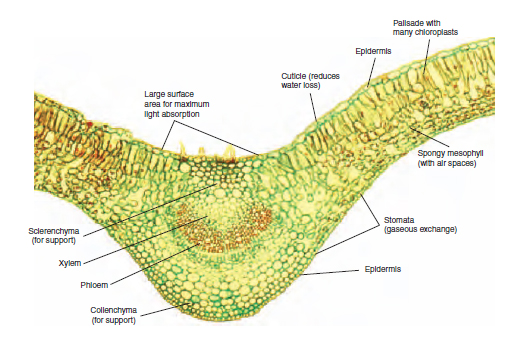
The leaf is the main organ for photosynthesis in the plant, and its cells are organized in a way that provides maximum efficiency. The upper epidermis is transparent enough to allow the transmission of light into the lower leaf tissues. The sausage-shaped palisade mesophyll cells are packed together, pointing downwards, under the upper epidermis. The sub-cellular chloroplasts within them carry out the photosynthesis process. The absorption of light by chlorophyll occurs at one site and the energy is transferred to a second site within the chloroplast where it is used to build up carbohydrates, usually in the form of insoluble starch. The spongy mesophyll, below the palisade mesophyll, has a loose structure with many air spaces. These spaces allow for the two-way diffusion of gases. The carbon dioxide from the air is able to reach the palisade mesophyll; and oxygen, the waste product from photosynthesis, leaves the leaf. The numerous stomata on the lower leaf surface are the openings to the outside by which this gas movement occurs. The numerous small vascular bundles (veins) within the leaf structure contain the xylem vessels that provide the water for the photosynthesis reaction. The phloem cells are similarly present in the vascular bundles, for the removal of sugar to other plant parts. Figure 8.8 shows the structure of the leaf and its relevance to the process of photosynthesis. A newly expanded leaf is most efficient in the absorption of light, and this ability reduces with age. The movement of the products of photosynthesis is described in Transport in the plant. Pollution Gases in the air, which are usually products of industrial processes or burning fuels, can cause damage to plants, often resulting in scorching symptoms of the leaves. Fluoride can accumulate in composts and be present in tap water, so causing marginal and tip scorch in leaves of susceptible species such as Dracaena and Gladiolus. Sulphur dioxide and carbon dioxide may be produced by faulty heat exchangers in glasshouse burners, especially those using paraffin. Scorch damage over the whole leaf is preceded by a reddish discolouration. Respiration In order that growth can occur, the food must be broken down in a controlled manner to release energy for the production of useful structural substances such as cellulose, the main constituent of plant cell walls, and proteins for enzymes. This energy is used also to fuel cell division and the many chemical reactions that occur in the cell. The energy requirement within the plant varies, and reproductive organs can respire at twice the rate of the leaves. Also, in apical meristems, the processes of cell division and cell differentiation require high inputs of energy. In order that the breakdown is complete, oxygen is required in the process of aerobic respiration. A summary of the process is given in Table 8.2.
It would appear at first sight that respiration is the reverse of photosynthesis. This supposition is correct in the sense that photosynthesis creates glucose as an energy-saving strategy, and respiration breaks down glucose as an energy releasing mechanism. It is also correct in the sense that the simple equations representing the two processes are mirror images of each other. It should, however, be emphasized that the two processes have two notable differences. The first is that respiration in plants (as in animals) occurs in all living cells of all tissues at all times in leaves, stems, flowers, roots and fruits. Photosynthesis occurs predominantly in the palisade mesophyll tissue of leaves. Secondly, respiration takes place in the torpedo-shaped organelles of the cell called mitochondria. Photosynthesis occurs in the oval-shaped chloroplasts. Details of biochemistry, beyond the scope of this book, would reveal how different these processes are, in spite of their superficial similarities. In the absence of oxygen, inefficient anaerobic respiration takes place and incomplete breakdown of the carbohydrates produces alcohol as a waste product, with energy still trapped in the molecule. If a plant or plant organ such as a root is supplied with low oxygen concentrations in a waterlogged or compacted soil, the consequent alcohol production may prove toxic enough to cause root death. Over-watering, especially of pot plants, leads to this damage and encourages damping-off fungi. Storage of plants The actively growing plant is supplied with the necessary factors for photosynthesis and respiration to take place. Roots, leaves or flower stems removed from the plant for sale or planting will cease to photosynthesize, though respiration continues. Carbohydrates and other storage products, such as proteins and fats, continue to be broken down to release energy, but the plant reserves are depleted and dry weight reduced. A reduction in the respiration rate should therefore be considered for stored plant material, whether the period of storage is a few days, e.g. tomatoes and cut flowers, or several months, e.g. apples. Attention to the following factors may achieve this aim:
|
||||||||||||||||||||||||