Soil components
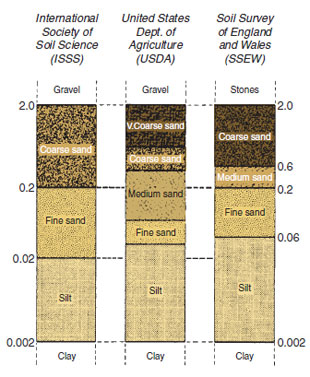
ContentThe solid parts of soils consist of mineral matter derived from rocks and organic matter derived from living organisms. Levels and characteristics of organic matter are dealt with in Chapter 18. Most soils have predominantly mineral particles that vary enormously in size from boulders, stones and gravels down to the smaller soil particles (‘fine earth’); sand, silt and clay. Particle size classes There is a continuous range of particle sizes, but it is convenient to divide them into classes. Three major classification systems in use today are those of the International Society of Soil Science (ISSS), United States Department of Agriculture (USDA) and the Soil Survey of England and Wales (SSEW). These are illustrated in Figures 17.8 and 17.9. In this text the SSEW scale used by the Agricultural Development and Advisory Service of England and Wales (ADAS) is adopted. In each case, soil is considered to consist of those particles that are less than 2 mm in diameter. The silt and clay particles are sometimes referred to as ‘fines’.
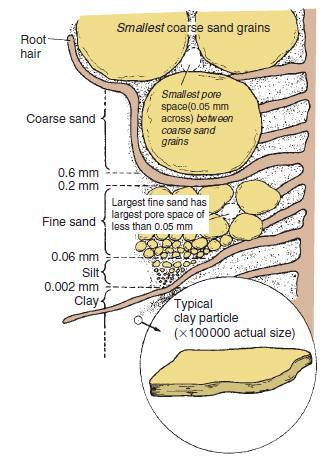
Sand Sands are gritty to the touch; even fine sand has an abrasive feel. Sand is mainly composed of quartz. (Although any particle of this size is a sand grain, it is most often quartz because, unlike other minerals, it resists weathering.) The shape of the particles varies from the rough and angular sand to more weathered, rounded grains. They are frequently coated with iron oxides, giving sand colours from very pale yellow to rich, rusty brown. Silver sand has no iron oxide covering. Chemically most of the sand grains are inert; they neither release nor hold on to plant nutrients and they are not cohesive. The influence of sand on the soil is mainly physical, and as such the size of the particles is the important factor. As the particles become smaller and the volume of individual grains decreases, the surface area of the same quantity of sand becomes greater (see Figure 17.10). Sand grains are non-porous so their water-holding properties are directly related to their surface areas. It can be readily seen that, since water will not flow through gaps less than about 0.05 mm in diameter, there are very big differences in the drainage characteristics of coarse and fine sands (Figure 17.9). Consequently, soils dominated by coarse sand are usually free draining but have poor water retention, whereas those composed mainly of unaggregated fine sand hold large quantities of water against gravity. The water held on all sand particles is readily removed by roots (see available water).
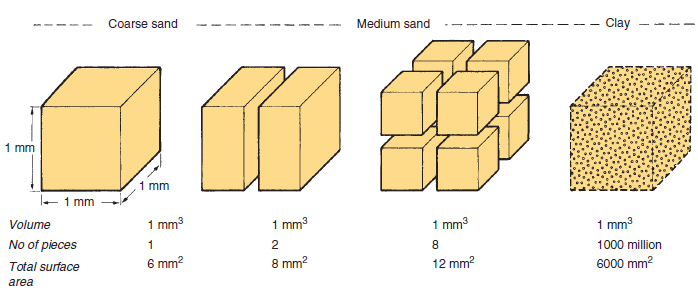
Clay There are many different clay minerals, e.g. kaolinite, montmorillinite, vermiculite, and mica, all of which are derived from rock minerals by chemical weathering. Most clay minerals have a layered crystalline structure and are platelike in appearance (Figure 17.9). The clay particles have surface charges that give clay its very important and characteristic property of cation exchange. The charges are predominantly negative which means that the clay platelets attract positively charged cations in the soil solution. These include the nutrients potassium, as K+; ammonia, as NH4+; magnesium, as Mg++; and calcium, as Ca++, as well as hydrogen and aluminium ions. These ions are held in an exchangeable way so that they remain available to plants, but are prevented from being leached unless displaced by other cations. The greater the cation exchange capacity, the greater the reserves of cations held this way. Hydrogen and aluminium cations make the soil acid. The other cations Ca++, Mg++, K+ and Na+ are called bases and make soils more alkaline (see soil pH). The proportion of the cation exchange capacity occupied by bases is known as its percentage base saturation. A soil’s buffering capacity, i.e. its ability to resist changes in soil pH, also depends on these surface reactions. The presence of high levels of exchangeable aluminium and hydrogen means that very large quantities of calcium, in the form of lime, are required to raise the pH of acid clays. In contrast, only small quantities of lime are needed to raise the pH of a sand by the same amount (see liming). The clay particles are so small that the minute electrical forces on the surface become dominant (Figure 17.10); thus clay and water mixtures behave as colloids (see Table 17.1). This gives clay soils properties of cohesion, plasticity, shrinkage and swelling. The small particles can pack and stick together very closely and in a continuous mass they restrict water movement. The water-holding capacity of clay-dominated soils is very high because of the large surface areas and because many of the particles are porous. However, a high proportion of the water is held too tightly for roots to extract. Moist balls of clay are plastic, i.e. can be moulded. On drying, they harden and some shrink. In the soil, the cracks that form on shrinking become an important network of drainage channels. The cracks remain open until the soils are re-wetted and the clay swells. Humus and calcium appear to combine with clay in such a way that when the combination dries, extensive cracking occurs and favourable growing conditions result. Some clays are non- shrinking and are consequently more difficult to manage although they present less problems with regard to building foundations. Characteristics of small particles Taking a cube and then cutting it up into smaller cubes readily demonstrates the relationship between volume and surface area (see Figure 17.10). While the total volume is the same in all the small cubes compared with the original large cube, the sides of each are smaller, but the total surface area is much greater because new surfaces have been exposed. Many soil particle characteristics are directly related to particle size and in
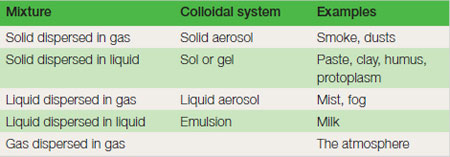
Note that as a particle is sub-divided the total surface area increases; the surface area doubles each time the sides of the individual pieces are reduced by half. In particles that are less than 0.001 mm in diameter, which includes most clay, colloidal properties are observed; most notably the properties of their surface dominate their chemical behaviour. Colloids are mixtures that are in permanent suspension. Water based colloids, such as clay, are ‘runny’ when mixed with plenty of water (a ‘sol’) but with less water they are stiff and jelly-like (a ‘gel’), e.g. paste. As they dry, these mixtures become sticky and eventually hard. Many natural materials such as gelatin, starch, gums and protoplasm (mainly protein and water) are colloidal. Silt Most in this size range are inert and non-porous like sands, but many particles, including felspar fragments, have the properties of clay. Soils dominated by silt do have a small cation exchange capacity, but in the main they behave more like a very fine sand. They have very good water-holding capacity and plants can take up a high proportion of this water. When wetted they have a distinctly soapy or silky feel. Silt soils are made up of particles that readily pack closely together, but have little ability to form stable crumbs (see soil aggregates). This makes them particularly difficult to manage. Stones and gravel Particles bigger than sand are commonly known as grit, gravel, pebbles, cobbles and boulders, according to size and shape. The effect of stones on cultivated areas depends on the type of stone, size and the proportion in the soil. The presence of even a few stones larger than 20 cm prevents cultivation. Stones in general have detrimental effects on mechanized work; ploughshares, tines and tyres are worn more quickly, especially if the stones are hard and sharp such as broken flint. Stones interfere with drilling of seeds and the harvesting of roots. Close cutting of grasses is more hazardous where there are protruding stones. Mole draining becomes less effective in stony soils and large stones make it impossible. Stones can accumulate in layers and become interlocked to form stone pans. In very gravelly soils the water-holding capacity is much reduced and the increased leaching leads to acid patches. Nutrient reserves are also reduced by the dilution of the soil with inert material. However, stones can help water infiltration, protect the surface from capping, and check erosion by wind or water. |