Biofertilizer Mass Cultivation of Microbial Inoculants
Rhizobium
Species of Rhizobium are kept in different specialization groups. Inoculum of different strains are prepared separately and cultivated on large scale, as required. Strains of Rhizobium sp. are grown in Yeast Extract Mannitol (YEM) broth in a small or large container as needed. Chemical composition of YEM broth is as below :
| Yeast extract | 1g |
| Mannitol | 10g |
| K2HPO4 | 0.5 g |
| Mg SO4 7H2O | 0.2 g |
| NaCl | 0.1 g |
| Distilled Water | 1000 ml |
| pH | 6.5 - 7.0 |
Methods of Cultivation
Following are the steps of mass cultivation of Rhizobium. (a) sterilize the growth medium and inoculate with broth of mother culture prepared in advance, (b) incubate for 3-4 days at 30 - 32°C, (c) test the cultures for its purity and transfer to a large fermenter, wait for 4-9 days for bacterial growth (for good bacterial growth make the device for its aeration), (d) allow to grow the bacteria either in a large fermenter containing broth or in small flasks as per demand, (e) check the quality of broth, (f) blend the broth with sterile carrier e.g. peat, lignite, farmyard manure and charcoal powder, (g) pack the culture in polyethylene bags and keep at 25°C, (h) check the quality of carrier culture, (i) store at 4°C in a controlled-temperature room, and (j) supply to farmers.
During the blending of broth a variety of carriers are used, for example, peat, lignite, farmyard manure, charcoal powder, etc. In India powdered farmyard manure and charcoal powder are good carrier and an alternative to peat and lignite. Good quality of carrier culture is that which contains sufficient amount of rhizobial cells i.e. 1000 x 106 to 4000 x 106 rhizobia/g carrier. Seed inoculation with aqueous suspension of carrier culture during sowing has revealed the luxuriant nodulation and good yield of crops.
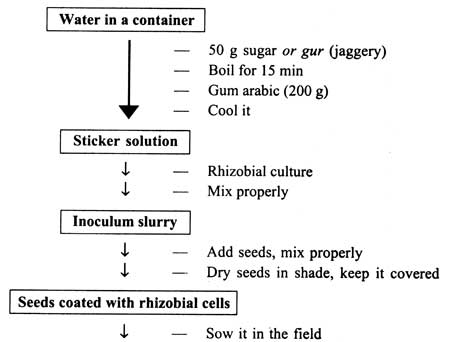
The steps of seed inoculation with rhizobial culture are given in Fig. 12.1. Dissolve 10 per cent sugar or gur (jaggery) in water by boiling it for some time. Leave the content to cool down. Gum arabic solution (10% ) may also be added to the solution. This serves as sticker for Rhizobium cells to seeds. Mix this carrier based culture of Rhizobium to form the inoculum slurry. For one hectare, 400 g charcoal based culture would be sufficient for mixing the seeds. Transfer the inoculum slurry on seeds and mix properly. The number of rhizobial cells/ seed should be between 105 to 106. Spread the seeds in shade for drying on cement floor or plastic sheets.
While using rhizobial cultures, certain precautions are taken into account. For example, use of culture before expiry date, use of small amount of pesticides when required, immediate sowing of seeds after mixing, etc. Seeds must be stored at 4°C when not used immediately to protect the rhizobial cells.
When soil has the adverse conditions such as dryness, acidity, excess fertilizers and pesticides, etc., the rhizobial cells are protected by adopting special method of inoculation. One of these methods is pelleting i.e. preparation of pelleted seeds. This method involves the procedure as described earlier. High amount of gum arabic (40%) or carboxymethylcellulose (20%) is added to the inoculum slurry before mixing with seeds. Finally, pelleting agent is mixed when inoclated seeds are moist (before seed drying ) to get the seeds evenly coated.
The commonly used pelleting agents are calcium carbonate, rock phoshate, charcoal powder, gypsum and betonite.
Effect of rhizobial culture on yield of different pulses, and subsequent crops grown after harvesting the pulse crops are given in Table 12.1 based on the study made under All India Co-ordinated Pulse Improvement Research Programme of I.C.A.R., New Delhi. Yield of pulse crops can be substantially increased by rhizobial inoculation. Legume crops get benefit from rhizobial symbiosis. In addition, certain amount of N is left over in soil which is taken by the other plants also.
Subba Rao and Tilak (1977) studied the effect of residual nitrogen of many legumes on the yield of subsequent crops of wheat or rice. They always found more yield of subsequent crops in Rhizobium inoculated fields than in uninoculated control.
Table 12.1. Response of different pulse crops to rhizobial cultures under different agroclimatic condition and residual effect on yield of subsequent crops.
| Crops | Location | Crop response (range in % increase in grain yield (q/ha) over UI control)* |
Yield of subsequent crops (% increase in yield over control, soil pH 7.3)** | |
| Crops | Yield % increase (q/ ha) over control |
|||
| Arhar (Cajanu cajan) |
Hisar, Haryana | 5 -25 | Wheat (Triticwn aestivum) |
UI-20.75 16.4 |
| Pantnagar, U.P. | 2-25 | RI-24.15 | ||
| S.K. Nagar, Gujarat | 9-21 | |||
| Sehore, M.P. | 13-29 | |||
| Rehari (Maharastra) | 3-40 | |||
| Chickpea (Cicer aritinum) |
Varanasi, U.P | 4-19 | Rice (Oryza sativa) |
UI-25.15 7.9 |
| Dholi, Bihar | 25-40 | RI-27.15 | ||
| Delhi | 18-28 | |||
| Hisar | 24-43 | |||
| Dohad, Gujarat | 33-67 | |||
| Sehore | 20-41 | |||
| Maharastra | 8-12 | |||
| Lentil (Lens culinaris) |
Pantnagar, U.P | 4-26 | Rice | UI-22.57 13.2 |
| Ludhiana, Punjab | No response | Rice | RI-25.55 | |
| Urd bean (Vigna munga) |
Pudukkotti, T.N. | 4-21 | Wheat | UI-20.75 2.4 |
| Dholi, Bihar | 11-29 | RI-21.25 | ||
| Pantnagar | ||||
| Pudukkotti, T.N. | ||||
Growth medium prepared for Rhizobium also supports the growth of A. chroococcum. Media used for mass cultivation of Azotobacter, Azospirillum and phosphate solubilizing bacteria differs in chemical composition (Table 12.2). Procedure for preparation of inoculants on large scale is similar to that of Rhizobium. Similarly, seed bacterization is also done as described earlier. The roots of transplantable crops e.g. vegetable) are dipped into aqueous suspension of carrier culture and then sown in fields.
Table 12.4 shows the effect of Azotobacter inoculant on yield of many crops. Tien et at (1979) have attributed that increased yield in pearlmillet obtained by inoculation of Azospirillum was due to the production of indoleacetic acid, gibberellins and cytokinin like substances by the bacterium and their subsequent effect on the plant.
Table 12.2. Composition of media for mass cultivation of bacteria (g/l).
| Chemical composition | Azotobacter chroococcum | Azospirillum | Bacillus megaterium, Pseudomonas striata |
| KH2 PO4 | 1.0 | 0.50 | - |
| MgSO4 7H2O | 0.50 | 0.10 | 0.10 |
| NaCl | 0.50 | 0.02 | - |
| KCL | - | - | 0.20 |
| MnSO4 .H20 | - | 0.01 | - |
| CaCO2 | 2.00 | - | - |
| KOH | - | 4.00 | - |
| (NH4)2 . SO4 | - | - | 0.50 |
| MnSO4 | - | - | 0.002 |
| FeSO4 | 0.10 | - | 0.002 |
| FeSO47. H2O | - | 0.05 | 5.00 |
| Ca3(Po4)2 | - | - | - |
| Na2MoO4 | - | 0.002 | 5.00 |
| CaCl2 | - | 0.01 | - |
| Sucrose | 20 | - | - |
| Glucose | - | - | 10 |
| Malic acid | - | 5.00 | - |
| Yeast extract | - | - | 0.5 |
| pH | - | 6.0-7.0 | - |
Field experiments conducted at IARI, New Delhi, have shown that upon seed inoculation of sorghum and cotton by A. chroococcum the yield was increased by 38 pet cent and 27 per cent respectively, whereas increase in yield of wheat was 10 per cent at the same conditions. These experiments suggest that small and marginal farmers can follow the seed bacterization and increase crop yield. Effects of these bacteria on yield of crops are given in Tables 12.3. and 12.4.
Table 12.3. Effect of seed inoculation with Azospirillum brasilense on grain yield (q/ha).
| Plant species * | Treatments | References | ||||
| Control (N-free) |
A. brasilense | Urea | Urea + A. brasilense |
|||
| 1. | Eleusine coracana (fingermillet) |
16.54 | 19.47 | 23.07 | 28.09 | Subba Roa et al. (1985) |
| 2. | Pennisetum americanum (pearlmillet) |
12.4 | 14.3 | 20.4 | - | Tilak and Subba Rao (1987) |
| 3. | Sorghum biocolor ** (sorghum var. CSH 5) |
19.23 | 22.78 | 30.48 | 31.05 | Tilak (1991) |
Table 12.4. Effect of Azotobacter inoculant on crop yield.
| Crops | % increase in yield over uninoculated control Azotobakterin * | Azotobacter chroococcum ** |
| Cotton | - | 6.7 -26.6 |
| Barley | 9.0 | - |
| Maize | 8.0 | 36.5 – 71.7 |
| Oat | 12.0 | - |
| Potato | 8.0 | - |
| Sorghum | - | 9.3 – 38.1 |
| Sugar beat | 7.6 | - |