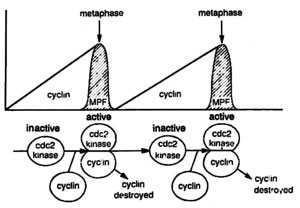
Fig. 8.6. Gradual increase in cyclin concentration leading to sudden appearance of MPF activity at metaphase, the midpoint of mitosis. Cyclin is then degraded and MPF activity drops. If cyclin degradation is stopped, the cells get stuck at mid-mitosis.
During the last decade, significant progress has been made in understanding the biochemical machinery that controls cell division. A clue to the biochemical events that accompany cell division
came in 1971, when in the immature frog eggs was discovered a factor called
maturation promoting factor or
MPF (a protein), which causes the eggs to undergo meiosis and divide, thus preparing for fertilization. Later, it was shown that MPF could also induce mitosis. It was shown that MPF activity fluctuates during the cell cycle, rising when the cell enters meiosis or mitosis and dropping sharply after the division is complete. A similar protein called
cyclin was discovered in sea urchin eggs and clam eggs. Like MPF, cyclin concentration was also found rising with cell division and fall drastically, when the cell division is completed. (Fig. 8.6) Later, cyclin was shown to be a part of MPF.

Fig. 8.6. Gradual increase in cyclin concentration leading to sudden appearance of MPF activity at metaphase, the midpoint of mitosis. Cyclin is then degraded and MPF activity drops. If cyclin degradation is stopped, the cells get stuck at mid-mitosis.
While the biochemists were busy in the study of proteins as above, the geneticists identified in yeast a series of mutations (discussed above) that interrupt cell cycle at various points. One of the genes identified through these mutations, was
cdc 2, which was found to code for a protein called p34
cdc2 a kinase responsible for phosphorylation of cyclin protein described above. By the end of 1988, it could be shown that cyclin and kinase are two components of MPF. The protein MPF was isolated and purified and was shown to consist of a cyclin (45,000 daltons) and a kinase or p34
cdc2 (34,000 daltons). These two proteins (particularly p34
cdc2 also described as
cdc 2 kinase) have been found in a wide range of organisms ranging from yeast to sea urchin,
Drosophila, frog and even humans. While the level of p34
cdc2 is constant during the cell cycle, that of cyclin rises at two points in the cell cycle (G1 cyclins accumulate upto 'start' point and M cyclins accumulate upto metaphase). Soon after reaching their peak values, cyclins undergo degradation, failing which the cell division can not proceed further.
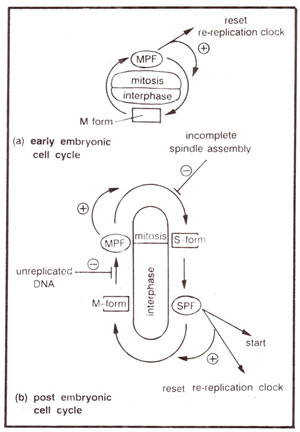
Fig. 8.7. Models of the early embryonic and post embryonic ceil cycles : (a) In the early embryonic cell cycle only, p34cdc2 is present in only one form (M form); block to re-replication of DNA is removed by activation of MPF. (b) In post embryonic cell cycle, p34cdc2 exists in two forms (M form and S form) giving MPF and SPF. Feedback controls regulate the activation and inactivation of MPF to ensure that mitosis does not begin till DNA replication is complete, and anaphase does not begin until assembly of spindle is finished.
It was later shown that cyclin synthesis itself is sufficient to activate the p34
cdc2 component of MPF and makes frog eggs undergo mitosis. Further, the cyclins accumulate in each interphase and their destruction was necessary (but not sufficient) to shut off p34
cdc2 component of MPF thus permitting completion of cell-division through inactivation of MPF. If degradation of cyclin during cell division is blocked, cells get struck in mid-mitosis and can not complete the cell division. Another gene product required for inactivation of MPF is p13
suc1 (product of fission yeast gene
sue 7).
iThe cells deleted for this gene are unable to inactivate MPF. It has also been shown that pl3
suc1 binds to p34
cdc2 component of MPF.
Cyclins described above can be of three types,
mitotic cyclins (cyclin B) found during M phase, S-phase cyclins (cyclin A), found during S-phase and
Gl cyclins (called Cln in yeast and cyclins C, D, E and F in vertebrates) found during G1 phase of the cell cycle. In combination with mitotic cyclins, p34
cdc2 can form active protein kinase, known as growth associated H1 kinase or MPF, which induces the events of mitosis.
Alternatively p34
cdc2 can combine with Gl cyclins, and catalyze passage through start (an event that commits the cell to initiate DNA replication). By analogy, this p34
cdc2-G
1 cyclin complex is described as
start promoting factor (SPF). Thus the alternation of replication (S phase) and segregation (M phase) is actually brought about by alternating activation of
p3Acdc2 component of the SPF and MPF activities. How is this alternation of activities actually brought about? One hypothesis suggests that Gl cyclins and mitotic cyclins are synthesized at different points in the cell cycle. However, it has also been shown that modified mitotic cyclins can also perform the function of Gl cyclins.
With the help of certain mutants, which replicate their DNA twice in fission yeast, it was also concluded that immediately after M phase,
cdc 2 molecule (or p34
cdc2) is in a form, described as S-form, which can be converted into active SPF (due to linkage with Gl cyclins) and induce DNA replication. At some point after start, but before the completion of DNA replication, the p34
cdc2 molecule is converted into a form described as M form, which can be converted into active MPF and not into active SPF. The formation of active MPF guarantees completion of S phase. During the passage through mitosis, M form of
p3Acdc2 again gets converted into S form (Fig. 8.7). The S and M forms of
p3Acdc2 are distinguished from each other due to post-translational modification of this protein.

Fig. 8.7. Models of the early embryonic and post embryonic ceil cycles : (a) In the early embryonic cell cycle only, p34cdc2 is present in only one form (M form); block to re-replication of DNA is removed by activation of MPF. (b) In post embryonic cell cycle, p34cdc2 exists in two forms (M form and S form) giving MPF and SPF. Feedback controls regulate the activation and inactivation of MPF to ensure that mitosis does not begin till DNA replication is complete, and anaphase does not begin until assembly of spindle is finished.
During the cell cycle, besides the reversible phosphorylation of cyclin proteins, p34
cdc2 also undergoes phosphorylation and dephosphorylation, which regulate its activity. After passing through
'start', the cells synthesize mitotic cyclins (cyclin B), which accumulate and bind to
p3Acdc2 This complex is initially inactive, due to associated phosphorylation of
tyrosine 15 (tyr-15) of p34
cdc2 (tyr-15 phosphorylation inhibits kinase activity). This inactive complex undergoes further phosphorylation at
threonine 160 (thr-160), which is necessary for MPF activity, but is not sufficient to overcome the inhibitory effect of tyrosine phosphorylation. Subsequent removal of tyrosine phosphate from
\s3Acdc2 during G2 activates MPF and leads to induction of mitosis (M phase). In fission yeast, tyrosine kinase is the product of
wee1 gene, whereas phosphatase is the product of
cdc25.
Active MPF activates tyr-phosphatase and inhibits tyr-kinase. This leads to entry into mitosis. MPF induces chromosome condensation, nuclear envelope break down and assembly of spindle. MPF also activates enzymes that conjugate ubiquitin to cyclin leading to cyclin degradation, which renders MPF inactive. Cyclin degradation and MPF inactivation lead to chromosome segregation, chromosome decondensation, nuclear reformation and cytokinesis.








