Sodium in Various Plant Species
One has to be cautious about interpreting concentrations expressed on the basis of different units (30,185). A tissue dry weight basis is often used in the agricultural literature, but conveys no information about the osmotic effects of solutes such as sodium ions or about changes in other dry weight components such as chloride in euhalophytes. Thus, ash-free dry weight might be a more appropriate basis for measuring concentrations. Using a fresh-weight basis does not facilitate the proper assessment of osmotic contributions of solutes, nor does it provide information about changes in the amount of solute independent of the amount of solvent (water). Expressing concentrations on a plantwater basis, or as measured concentrations in cell sap, does convey information about the osmotic effects of solutes, but does not allow a distinction to be made between osmotic adjustment sensu stricto and changes in the water content of the tissue. An example is given in Reference (185), where sodium concentrations in the roots and shoots of mammoth wildrye (Leymus sabulosus Tzvel.) are compared as concentrations in sap or as concentrations per kilogram dry weight. The conclusion about whether there are higher concentrations of sodium in the roots or shoots is reversible depending on which units are used.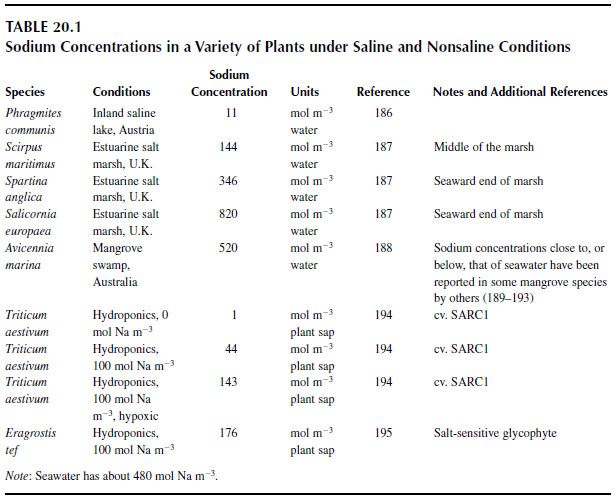 |
Table 20.1 shows the concentrations of sodium in the healthy shoots of different species. Under nonsaline conditions, the sodium concentrations in most plant tissues are a few moles per cubic meter plant water at most. As external salinity is increased, the amount of sodium within the plant increases, but the rate at which this increase occurs varies from slow in wheat to very rapid in tef, a salt-sensitive glycophyte with little ability to control the influx of sodium. Halophytes accumulate substantial amounts of sodium, but are able to tightly control this accumulation at salinities close to or below that of seawater.
In conclusion, sodium is essential only for some C4 species, but is undoubtedly beneficial to the growth of euhalophytes. It may stimulate the growth of some species with an evolutionary history in saline environments, and even of apparently totally glycophytic species under certain conditions. Whether there is a need to reclassify sodium as a 'functional' nutrient is open to debate. These considerations are, however, of minor importance compared with the problems caused by the secondary salinization of agricultural land.




