Therapeutic Supplements
Microalgae are a unique source of high-value compounds and systematic screening for therapeutic substances, particularly from cyanobacteria, has received great attention. Among cyanobacteria Spirulina sp. has undergone numerous and rigorous toxicological studies that have highlighted its potential therapeutic applications in the area of immunomodulation, anticancer, antiviral, and
cholesterol reduction effects.
A number of extracts were found to be remarkably active in protecting human lymphoblastoid T-cells from the cytopathic effects of HIV infection. Active agents consisting of sulfolipids with different fatty acid esters were isolated from Lyngbya lagerheimii and Phormidium tenue. Additional cultured cyanobacterial extracts with inhibitory properties were also found in Phormidium cebennse, Oscillatoria raciborskii, Scytonema burmanicum, Calotrix elenkinii, and Anabena variabilis. A protein called cyanovirin, isolated from an aqueous cellular extract of Nostoc elipsosporum prevents the in vitro replication and citopathicity of primate retroviruses.
Cryptophycin 1, an active compound isolated from Nostoc strain GSV224, exerts antiproliferative and antimitotic activities by binding to the ends of the microtubules, thus blocking the cell cycle at the metaphase of mitosis. Cryptophycin 1 is the most potent suppressor of microtubule dynamics yet described. Research has been focused on its potent antitumor activity and a synthetic analogue, cryptophycin-52, is at present in Phase II clinical trials by Eli Lilly & Co, Inc. (Indianapolis, IN). Other studies using water soluble extracts of cyanobacteria have found a novel sulfated polysaccharide, calcium spirulan to be an antiviral agent. This compound appears to be selectively inhibiting the penetration of enveloped viruses into host cells, thereby preventing the replication. The effect was described for many different viruses like herpex simplex, measles, and even HIV-1.
Among eukaryotic microalgae, a glycoprotein prepared from Chlorella vulgaris culture supernatant exhibited protective activity against tumor metastasis and chemotherapy-induced immunosuppression in mice.
Extracts from several macroalgae may prove to be a source of effective antiviral agents; although the tests have been either in vitro (in test-tubes or similar) or on animals, with few advancing to trials involving people. A notable exception is Carraguard, a mixture of carrageenans similar to those extracted from Irish moss. Carraguard has been shown to be effective against human immunodeficiency virus (HIV) in vitro and against herpes simplex virus in animals. Testing has advanced to the stage where the international research organization, the Population Council, is supervising large-scale HIV trials of Carraguard, involving 6000 women over 4 yr. Extracts from the brown macroalgae, Undaria pinnatifida, have also shown antiviral activity; an Australian company is involved in several clinical trials, in Australia and the U.S., of such an extract against HIV and cancer. The Population Council’s trials against HIV involve the vaginal application of a gel containing carrageenan. Because antiviral substances in macroalgae are composed of very large molecules, it was thought they would not be absorbed by eating macroalgae. However, it has been found in one survey that the rate of HIV infection in macroalgae-eating communities can be markedly lower than it is elsewhere. This has led to some small-scale trials in which people infected with HIV ate powdered Undaria, with a resulting decrease of 25% in
the viral load.
Fucoxanthin, a carotenoid commonly distributed in brown algae, such as U. pinnatifida, Scytosiphon lomentaria, Petalonia binghamiae, and Laminaria religiosa, is a potent drug candidate and can be utilized as an excellent supplement like astaxanthin, because it acts as an antioxidant and inhibits GOTO cells of neuroblastoma and colon cancer cells. Recently, the apoptosis activity against HL-60 (human leukema) and Caco-2 (cancer colon) cells has been reported for fucoxanthin.
Among the microalgal high-value compounds there are carotenoids such as β-carotene, astaxanthin, PUFA such as DHA and EPA, and polysaccharides such as b-glucan. Carotenoids by their quenching action on reactive oxygen species carry intrinsic anti-inflammatory properties, PUFA exhibit antioxidant activity and polysaccharides act as immunostimulators.
A major bottleneck in the explotation of microalgal biomass for the production of high-value compounds is low productivity of the culture, both in terms of biomass and product formation. A fundamental reason for this is slow cell growth rates owing to inefficient use of strong light. Furthermore, most microalgal products are secondary metabolites that are produced when growth is limited. A solution to this bottleneck could be to milk the secondary metabolites from the microalgae. This involves continuous removal of secondary metabolites from cells, thereby enabling the biomass to be reused for the continuous production of high-value compounds.
Recently, a new method was developed for milking β-carotene from Dunaliella salina in a twophase bioreactor. In this technique, cells are first grown under normal growth conditions and then stressed by excess light to produce larger amounts of β-carotene. At this stage, the second, biocompatible organic phase is added and the β-carotene is extracted selectively via continuous recirculation of a biocompatible organic solvent (lipophilic compound) through the aqueous phase containing the cells. Because the cells continue to produce β-carotene, the extracted product is continuously replaced by newly produced molecules. Therefore, the cells are continuously reused and do not need to be grown again. In contrast to existing commercial processes, this method does not require the harvesting, concentrating, and destroying of cells for extraction of the desired product. Furthermore, purification of the product is simple owing to the selectivity of the extraction process. The general application of this process would facilitate the commercialization of microalgal biotechnology and development of microalgal products.
The properties of the cell membrane play an important role in the contact between biocompatible lipophilic solvents and hydrophobic parts of the cell membrane might be prevented by presence of a cell wall or hydrophilic parts of the outer membrane. Physiological properties of the cells, such as their capacity for continuous endo- and exocytosis, might also play a role in the milking process. Other considerations are the location and way in which the product accumulates inside the cells and the function of that product inside the cells. A product like chlorophyll would be difficult to extract owing to its location in thylakoid membranes and because it is bound strongly to other cell components. The extraction of a product with a protective effect on the cells (e.g., β-carotene) will enhance its synthesis. The milking process can be applied also to other algae and other products besides D. salina, for example in Haematococcus pluvialis for the recovery astaxanthin, and marine microalgae for PUFA.
In addition to its use in aquaculture (e.g., to give salmon a pink color), astaxanthin has also been described as having nutraceutical importance related to free-radical scavenging, immunomodulation, and cancer prevention. H. pluvialis can produce and accumulate astaxanthin to concentrations of 1–8% of the dry weight. This concentration within the cell would make milking of H.
pluvialis more successful compared with D. salina. However, cultivation of H. pluvialis is more complex than D. salina, and productivity is lower. Furthermore, extraction, purification, and concentration are a heavy burden on the production cost. As the final product cost is also sensitive to algal productivity and duration of the growth period, this process is not economically feasible at present.
PUFAs are gaining increasing importance as valuable pharmaceutical products and ingredients of food owing to their beneficial effect on human health. DHA (22 : 6 v3) and EPA, (20 : 5 v3), in particular, are important in the development and functioning of brain, retina, and reproductive tissues both in adults and infants. They can also be used in the treatment of various diseases and disorders, including cardiovascular problems, a variety of cancers, and inflammatory disease. At present, PUFAs are produced commercially from fish oil, but this is an insufficient source of these products and microalgae provide an optimal lipid source of PUFAs.
The heterotrophic marine dinoflagellate Crypthecodinium cohnii has a lipid content greater than 20% dry weight and is known for its ability to accumulate fatty acids that have a high fraction (30–50%) of DHA. Lipids are important components of algal cell membranes but also accumulate in globules in other parts of the cells. Microalgal growth and fatty acid formation is affected by medium composition and environmental conditions (e.g., carbon sources). Lipid production occurs under growth-limiting conditions; during linear growth, the cells are stressed owing to nutrient limitation and therefore produce more lipids. Also the concentration of DHA, hence the lipid quality, is negatively affected by increases in lipid concentration. The highest quality lipid it obtained when glucose is used as the carbon source, and when the cell concentration and lipid content of the cells are the lowest.
Milking can be used also for DHA production by C. cohnii. In this process, cells are first grown under the correct conditions for growth, after which they are stressed to produce higher concentrations of DHA. A biocompatible organic solvent is added during the DHA production stage to extract the product. This process enables the production of high-quality lipid, thereby reducing extraction and purification costs. Furthermore, higher amounts of DHA are produced by substitution of extracted lipids by newly synthesized lipid, increasing the productivity of the system.
Microalgae represent also one of the most promising EPA producers, as the purification of this PUFA from fish oil, which remains the main commercial source of EPA, involves many drawbacks. Many Eustigmatophyceae, such as Nannochloropsis sp. and Monodus subterraneus, and Bacillariophyceae species contain a considerable amount of EPA. An EPA production potential has been found in the genus Nitzschia (especially N. alba and N. laevis). It was reported that the oil content of N. alba was as high as 50% of cell dry weight and the EPA comprises 4–5% of the oil. N. laevis could utilize glucose or glutamate as single substrate for heterotrophic growth, and the cellular EPA content of the alga in heterotrophic conditions was also higher than that in photoautotrophic conditions suggesting that this diatom is a good heterotrophic EPA producer.
The bioprocess engineering aspect of heterotrophic EPA production by N. laevis has been extensively studied. Major achievements include:
- Optimization of various medium components (including silicate, glucose, nitrogen sources, salts and trace elements) and environmental factors (including pH, temperature) for the alga culture
- Investigation of detailed physiological behavior of the alga (cell growth, nutrient consumption, fatty acid compositions, etc.) by a continuous culture (the dilution rate and glucose concentration in the feed medium were optimized in terms of EPA productivity and glucose utilization efficiency)
- Development of high cell density and high productivity techniques, which led to an EPA yield of 1112 mg l-1 and an EPA productivity of 174 mg l-1 day-1, both of which are the highest ever reported in microalgal cultures.
Microalgae produce many different types of polysaccharides, which may be a costituent of the cell wall as in unicellular red algae as Porphyridium sp. and Rhodella sp., or be present inside the cell, as in the Euglenophyceae. The polysaccharides of Rhodophyta are highly sulfated and consist mainly of xylose, glucose, and galactose. These compounds selectively inhibit reverse transcriptase (RT) enzyme of human immunodeficiency virus (HIV) and its replication in vitro.
Rodents fed with a diet supplemented with biomass and polysaccharides derived from Porphyridium results in a decrease in blood cholesterol concentration (by 22% and 29%, respectively) and triglyceride levels, increased feces weight (by 130% and 196%, respectively) and bile acid excretion (5.1- and 3.2-fold or more). Moreover, algal biomass or polysaccharide increased the
length of both the small intestine (by 17% and 30%, respectively) and the colon (by 8.5% and 32%, respectively).
Paramylon is the term used for the reserve polysaccharide of Euglena and euglenoids in general. Its granules appearing in various locations inside the cell; in many species they are scattered throughout the cytoplasm, but others can be massed together or few, but large, and located in a fairly constant position. Their shape and size differ markedly, and together with their distribution inside the cell, represent a taxonomic feature. Paramylon consists of b-1,3-glucan, a linear polysaccharide found in the cell walls of many bacteria, plants, and yeasts. It belongs to a group of naturally occurring polysaccharides such as lentinan, fungal glucans, sizofiran, and pachyman, which are considered bioactive compounds. Lentinan and sizofiran are known to inhibit the growth of
various tumors, whereas fungal glucans are used clinically for their stimulatory effect on the immune system, in particular on the macrophages. The main interest in b-glucans stems from their ability to act as nonspecific immune system stimulants, by binding to a specific site on monocytes/macrophages and granulocytes.
They have been successfully used in aquaculture to strengthen the nonspecific defence of many important species of fishes and shrimps by injection, immersion, or in the feed. Sulfated derivatives of Euglena paramylon, in particular, have shown anti-HIV (human immunodeficiency virus) activity. Moreover, it has been suggested that this polysaccharide has a cholesterol-lowering effect when incorporated in the diet of either humans or animals, and moderates the postprandial blood glucose and insulin response in humans. Since Euglena gracilis can accumulate large quantities of paramylon when grown in the presence of an utilizable carbon source, it could represent an alternative source of this compound to Saccharomyces cerevisiae (baker’s yeast), which is currently exploited industrially for its extraction. Moreover, Euglena has been investigated as a potential protein source, and a promising dietary supplement due to its content of highly nutritious proteins and PUFAs, and to the simultaneous production of antioxidant compounds, such as β-carotene, vitamin C, and vitamin E.
Table 7.7 summarizes commercially exploited algae and the corresponding nutraceutical.
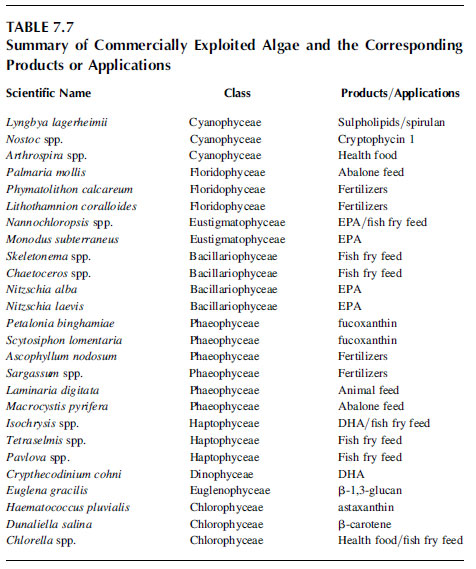
TABLE 7.7 Summary of Commercially Exploited Algae and the Corresponding Products or Application





