Cryobiology
These materials are stored at low temperature, due to which growth-rate of cells retards; consequently biological activities are conserved for long time.
Cryobiology deals with the study of metabolic activities and their responses in plant materials (and animal ceils) stored at low temperature (- 196°C) by using liquid nitrogen in the presence of cryoprotectants. Dodds and Roberts (1985) have discussed 3 principal methods used for growth suppression in plant tissue culture (i) the alteration of physiological conditions of culture i.e. temperature or gas composition within the vessel; (ii) changing the composition of basal medium e.g. using sub or supra-optimal concentrations of nutrients (some factors essential for normal growth may be either omitted or employed at a reduced level) and (iii) supplementing the medium with growth retardants (e.g. abscisic acid) or osmocegulatory compounds (e.g. mannital, sorbital, etc.).
Storage at reduced temperature has been very affective for tissue culture of most of the plant species such as potato, cassava (Manihot esculentum), pea (Pisum sativum), chickpea (Cicer arietinum), rice (Oryza sativa), wheat (Triticum vulgare), coconut (Cocos nucifera), oil palm (E. guineensis) strawberry (Fragaria vesca) and sugarcane (Saccharum officinarum) (Bajaj, 1987).
Content
» Difficulties in cryopreservation» Methods for cryopreservation
» Plant cell bank
» Pollen bank
» Achievements through cryopreservation
Difficulties in Cryopreservation
A number of reviews available during the last two decades illustrate the significant progress made in this field and also the outline of the existing problem (Witherr, 1980; Henshaw, 1982). The difficulties are (i) high specific feature of plant cells, such as their large size, strong vacuolization and abundance of water, (ii) cell damage during freezing and subsequent thawing caused by ice crystals formed inside the cells and by cell dehydration, and (iii) gradual formation of large crystals of more than 0.1mm whose facets rupture many cell membranes (Shimada and Ashahina, 1975). However, in the presence of cryoprotectants (the chemicals decreasing cryodestructJ0h) and reduced temperature, free water has enough time to leave the cells. Therefore, it can freeze on the crystal surface in the solution (Samygin, 1974). This results in marked dehydration and protoplast shrinkage (Muzur, 1977). Excessive time and degree of plasmolysis are the reasons of cell destruction during slow freezing, since they cause irreversible contractionofthe plasmalemma (Wiest and Steponkus, 1978).
The freezing-storage-thawing cycle is an external procedure consisting of the following basic stages:
(i) Selection of Materials. For selecting the plant materials a number of factors are taken into account ; the important ones are, nature and density of cells in the vials/ampules to be cryopreserved ; because the cryoability of the cell cultures depends on these. Young meristematic, highly cytoplasmic and small cells which are non-vacuolated and thin walled and in small aggregates, are good materials to be selected for this purpose. Cell density in vials or amples should be high, as it shows prolonged survival at high cell density.
(ii) Addition of Cryoprotectors. Cryoprotectors are the chemicals which decrease cryodestruction. These are sugars, glycols, sugar alcohols, alcohols, polyvinylpyrrollidone, polyethylene glycol (PEG), polyethylene oxide (PEO), dextrans, hydroxystarch, glycerine, sucrose, and some amino acids (e.g. proline). Bajaj (1987) has advised to use a mixture of two or three cryoprotectants at low concentrations rather than a single cryoprotectant at a high concentration as it could be toxic. During treatment, the cultures should be maintained in ice to avoid deleterious effects.
(iv) Storage in Liquid Nitrogen. If the cells are not stored at sufficiently low temperature, an additional injury to the cultures may be caused. The storage temperature should be such that it stops all metabolic activity and prevents biochemical injury (Bajaj 1987). Prolonged storage of frozen materials is possible only when the temperature is lower than -130°C. This can be simply achieved with the help of liquid nitrogen, which keeps the temperature -196°C. Popove (1988) stored the cultures of carrot cells for about 5 years by doing so.
(v) Thawing. Thawing is the process of releasing the vials containing cultures from the frozen state to elevate the temperature between 35 and 40°C. It should be done quickly but without overheating. As soon as the last ice crystals disappear, the vials are tranferred into a waterbath at 0°C (Popov, 1985).
(vii) Regeneration of Plantlets. The viable cells are cultured on non-specific growth media to regenerate into plantlets. Bajaj (1987) has given an extensive list of works on cryopreservation of cells, tissue, and organ culture of various plants e.g. potato, cassava, sugarcane, soybean, groundnut, carrot, cotton, citrus, coconut, etc.
Cryopreservation of genetic stock i.e. germplasm (or vegetatively propagated crops, recalcitrant producing plants, rare plant species, medicinal, horticultural and forest plants, and VAM fungi) is a novel approach for their conservation in liquid nitrogen on a long term basis. To achieve this goal, a plant cell bank ( = germplasm bank and cell cryobank) has been suggested by Bajaj (1977 a), Bajaj and Reinert (1977) and Popov (1985). Suggestions have also been made that germplasm bank should be attached to some of the International Research Institutes (e.g. IRRI) that would hold responsibility for the storage, maintenance, distribution (at national and international level), and exchange of these disease free germplasm of the important plants. Fig. 10.1. shows the potential and prospects of cryopreservation of plant cell, tissue and organ and establishment of germplasm bank.
Facilities for storage of genetic stock of plants can be developed in large sized cylinders (30-50 liters capacity) where liquid nitrogen does not require refilling for 6-8 months (Bajaj, 1987).
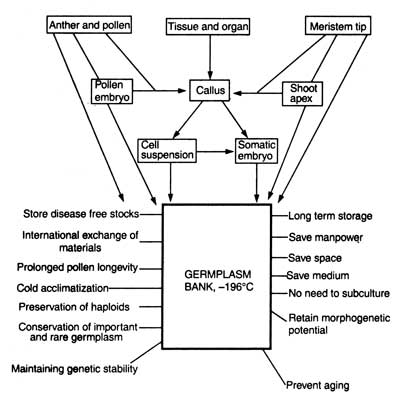
Fig. 10.1. Potentials and prospects of cryopreservation of plant cell, tissue and organ culture and establishment of .'Germplasm Bank' (after Bajaj, 1977a).
Pollen Bank Besides germplasm bank, the storage of pollen grains in liquid nitrogen and establishment of pollen bank have also been suggested to retain their viability for various lengths of time. The freeze storage of pollen would enable (i) hybridization between plants with flowers at different times, (ii) growth at different places, (iii) reducing the dissemination of diseases by pollination vectors, and (iv) maintenance of germplasm and enhancement of longevity (Bajaj, 1987).
Various forms of plant materials viz. cell suspensions clones, callii, tissues, somatic embryos, root/shoot tips propagules (tubers) pollen grains, etc. have been preserved in liquid nitrogen for prolonged time and tested for their survival and regeneration potential.
No doubt, in most of the cases, the cells/tissues, organs regenerated into plants. Bajaj (1987) has described a number of plant species that have been successfully cryopreserved. Some of the observations made are as below :
(i) Cryopreservation of cell lines : For example, cell suspensions (soybean, tobacco, dhatura, carrot) and somatic hybrid protoplasts (rice x pea, wheat x pea).
(ii) Cryopreservation of pollen and pollen embryos : For example, fruit crops, trees, mustard, carrot, peanut, etc.
(iii) Cryopreservation of excised meristems : For example, potato, sugarcane, chickpea, peanut, etc.
(iv) Cryopreservation of germplasm of vegetatively propagated crops: potato, sugarcane, etc.
(v) Cryopreservation of recalcitrant seeds and embryos: Large sized seeds that are shortlived and abortive, such as oil palm, coconut, walnut, mango and cocoa.




