Primary Metabolites
Organic Acids
A micro-organism grows in broth of the fermenter and during its trophophase of growth, produces organic acids. The organic acids are produced through the metabolisms of carbohydrates. They accumulate in broth of fermenter, wherefrom they are separated and purified.The organic acids are either the terminal products of EMP pathway (glycolysis), e.g. lactic acid and propionic acid or the products of incomplete oxidation of sugars (citric acid, itaconic acid and gluconic acid). A third type of product is also obtained from the dehydrogenation of alcohol in the presence of oxygen i.e. acetic acid (Riviere, 1977).
Organic acids offer a great potential for future development as they are manufactured on large scale. They are marketed relatively as pure chemicals or their respective salts. In 1881, for the first time calcium lactate was manufactured on a large scale by bacterial fermentation. Later on species of Penicillium and Aspergillus were discovered for the production of acids. In the present context production of citric acid is described it detail.
For the first time Scheele (1789) reported the isolation and crystallization of sour product from the lemon juice. However, Citrus fruits could contributed a small amount (1%) in the market. Now-a-days citric acid available in market comes through fermentation process.
Chemically, citric acid was synthesized from the glycerol (Grimaux and Adams, 1880). It was Wehmer (1893) who reported the wide occurrence of citric acid in the microbial metabolites. Molliard (1922) confirmed the accumulation of citric acid in the cultures of Aspergillus niger under the conditions of nutrient deficiency.
In the beginning, citric acid was recovered in small amount; however, it gave the basic concept of citirc acid production under the conditions of nutrient deficiency. A sufficient amount of biomass is produced under optimum nutrient levels. It is, however, obvious that production of mycelial mass or sporulation does not correspond to the production of citric acid in cultures (Lockwood, 1979).
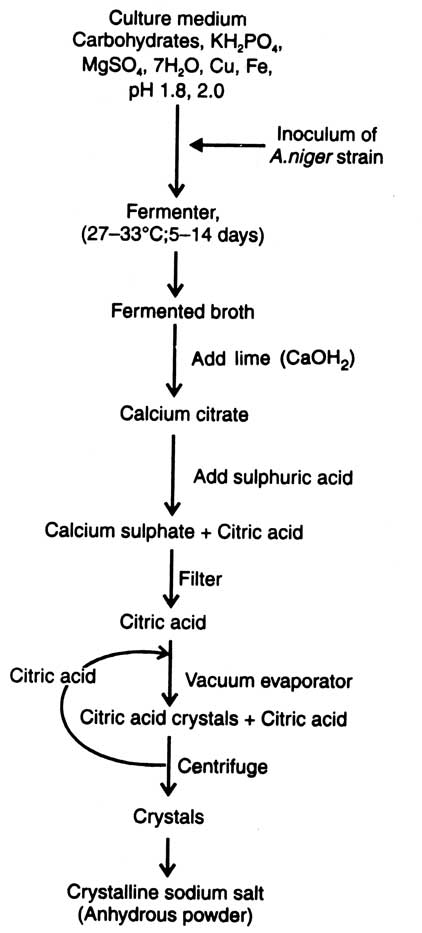
There are 3 methods for commercial production of citric acid: (i) Koji fermentation Process : This method is used in Japan which accounts for about one fifth of citric acid produced per annum. Strains of A. niger are used in this method. (ii) Liquid surface culture fermentation process : In this method, liquid cultures are inoculated with the spores of A. niger which germinate within 24 hours. Mycelia cover and float on whole surface of the solution. (iii) Submerged culture fermentation process : In this case the mycelia of A. japonicum grow in solution of about 15 cm depth in tanks. Citric acid produced in this way is inferior to that produced by liquid surface culture fermentation. Moreover, it is produced in low amount and at high cost. Methods of citric acid production are given in Fig. 15.2. For commercial production strains of A. niger are selected from the hybrids or mutants developed by certain procedure. The strains should be such that could produce not less than 80 g citric acid per 100 g glucose. For large scale production, continuous culture is not suitable. Therefore, a multitank system is required for continuous fermentation in any process in which cell growth and metabolic products occur at different times (Lockwood, 1979).
Culture medium contains carbohydrates, KH2PO4 (0.01-0.3%), MgSO4.7H2O (0.25%) and a few trace elements. Solutions of carbohydrates are high test cane syrup (concentrated cane juice), glucose or sucrose.
High test syrup can also be beet molasses or cane molasses. If substrate is molasses, ferrocyanide (20-150 mg/liter) can be added to culture medium, before sterilization, to precipitate excess iron (Clark, 1962).
Instead of iron, clarified molasses can be passed through an ion exchange resin. Under laboratory condition highest yields are obtained by using sucrose passed through an ion exchange resin. At this stage, it is necessary to add copper (0.1 - 0.50ppm) to the culture medium (Riviere, 1977). But the trace elements should be added only when fermenter is made up of steel or some impervious coating is applied (to ordinary fermenters) (Lockwood, 1979). pH of the culture medium is maintained to about 3.5 by adding ammonium nitrate (0.25%). It avoids the formation of oxalic and gluconic acids.
The culture medium is sterilized by passing through the pipes of a steam jacketed heat exchanger and cooled down to about 30°C in another exchanger.
Inoculum of A. niger strain is prepared by culturing it on nutrient agar medium. Stock cultures are maintained in culture tubes kept in refrigerator or in the form of lyophilized spores. When required spore suspensions are prepared in the sterile distilled water.
After inoculation, the culture solution must be aerated by bubbling the air to allow maximum growth for the fungus. Fermentation process is completed in about 5-14 days at 27-33°C. The fermented broth acts as the source of citric acid. Lime (Ca(OH)2) is added to allow precipitation of citric acid in the form of calcium citrate. Again the precipitate is treated with sulphuric acid to precipitate insoluble calcium sulphate. It is then filtered. The filtrate containing citric acid is purified by passing through column of carbon granules. The carbon granules should be treated with heat or washed with hydrochloric acid (HC1). Passing through ion exchange beds solution is demineralised. Which is then concentrated under vacuum to form crystals. Crystals are recovered by centrifugation and mother liquor is returned to evaporator to get the remaining crystals.
Biochemistry of fermentation
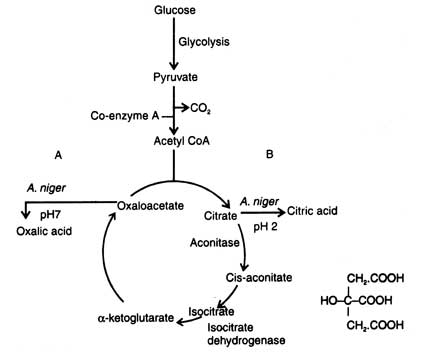
Citric acid is produced during trophophase as a result of interruption of tricarboxylie acid cycle (Krebs cycle). In A. niger about 78% sugar passes through EMP pathway and results in production of acetyl CoA which condenses with oxaloacetic acid to yield citric acid (Fig. 15.3). Further citric acid cycle is interrupted by inhibition of aconitase and isocitric dehydrogenase either by copper or hydrogen peroxide (Bruchmann, 1961). However, iron is an essential cofactor of these enzymes. The Cu and Fe in a ratio 0.3. : 2 (mg/liter) interrupt the activity of these enzymes at pH 2.0 (Lockwood, 1979)
Uses Of Organic Acids
(i) Acetic acid is used in food industry and research.
(ii) Fumaric acid is used in resins.
(iii) Gluconic acid is used in medicals as calcium -, iron - and potassium-gluconate, and in cleaning of bottles.
(iv) Lactic acid is used in food industry (fruit extracts, syrup and pickles), in dye mordant, in tanning, decaicifying skins, in plastics, and medicals as iron-and calcium lactates.
(v) Citric acid is used in food industry (e.g. fruit drinks, confectionery, jams, jellies, preserved fruits, candies, wines), pharmacy (e.g. blood transfusion, efferrescent products), cosmetics (e.g. astringent lotions, shampoos and hair setting fluids), and industries (e.g. electroplating, leather tanning, cleaning of pipes, reactivation of old oil wells).