Use of Permeabilised Mast Cells to Analyze Regulated Exocytosis
Cell permeabilisation allows manipulation of experimental conditions that is typical for in vitro assays while maintaining the integrity of cellular architecture. Permeabilised mast cells, both primary and cultured cell lines, such as rat basophilic leukemia (RBL-2H3) or human mast cells (HMC-1), have been used to investigate the mechanism of exocytosis, endocytosis, phospholipid metabolism, and cytoskeletal responses. After permeabilisation with a bacterial exotoxin, streptolysin- O (SL-O), cells lose their cytosolic components but retain intact and functional secretory vesicles. These cells provide a well-controlled system, ideal for reconstitution type experiments. Permeabilisation by SL-O is usually irreversible. Reversible permeabilisation of rat peritoneal mast cells can be achieved by exposure to the tetrabasic anion of ATP (ATP4-). This creates lesions (Cockcroft and Gomperts, 1979) due to interaction with a specific cell surface receptor (Tatham et al., 1988), the dimensions of which vary with increasing ATP4- concentration (Tatham and Lindau, 1990). The pores created by ATP4- are, however, always smaller than those due to SL-O. Combination of the two methods allows control of the extent of leakage of cytosolic factors (Koffer and Gomperts, 1989).
Holt and Koffer (2000) described the use of various recombinant mutants of small Rho GTPases in permeabilised mast cells. Other reviews of permeabilisation techniques have been published previously (Gomperts and Tatham, 1992; Larbi and Gomperts, 1996; Tatham and Gomperts, 1990). This article describes a "SL-O prebind" method for permeabilising glass-attached primary rat peritoneal mast cells. This method is based on the temperature-independent binding of SL-O to the plasma membrane and the temperature-dependent polymerisation of bound SL-O molecules required to form pores (Sekiya et al., 1996).
Percoll (1.13 ± 0.005g/ml stock) is from Amersham Biosciences (1 liter, Cat. No. 17-0891-01). 10× calcium/magnesium-free phosphate-buffered saline (PBS) with defined salt densities is from Gibco RBL (500 ml, Cat. No. 14200-067). Streptolysin-O (SL-O, Cat. No. 302) and sodium dithionite (Cat. No. 303, needed for reduction of SL-O) are from iTEST plus, Ltd. (Czech Republic). Other preparations of SL-O can be obtained from Sigma (e.g., Cat. No. S 5265), and SL-O can also be obtained from VWR Scientific, US (Cat. No. DF 0482-60, manufactured by Difco), but in our hands, the just-described reagent has given the most reproducible results. Sigma provides EGTA (Cat. No. E-4378), HEPES (Cat. No. H-4034), PIPES (Cat. No. P-1851), Tris (Cat. No. T-1503), glutamic acid, monopotassium salt (Cat. No. G-1501), 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide (Cat. No. M2133), dimethyl sulphoxide (DMSO, Cat. No. D-8779), Triton X-100 (Cat. X-100), goat serum (Cat. No. G9023), unconjugated succinyl-concanavalin A (SCA, Cat. No. L 3885), lysophosphatidylcholine (LPC, Cat. No. L-4129), paraformaldehyde (Cat. No. P-6148), and polyethylene glycol (molecular weight 3550, Cat. No. P-4338). Na2ATP (trihydrate, Cat. No. 519979), 100mM GTPγS solution (Cat. No. 1110 349), and digitonin (1g, Cat. No. 1500 643) are from Boehringer. Citric acid (Cat. No. 100813M), glycine (Cat. No. 101196X), solutions of 1M MgCl2 (Cat. No. 22093 3M) and 1M CaCl2 (Cat. No. 190464 K), and 18-mm2 coverslips, thickness 1 (Cat. No. 406/0187/23), are from BDH. Mowiol 4-88 is from Harco (Harlow Chemical Company Ltd.).
A moisture box is a small plastic box with a wellfitting lid lined with wet tissue paper. A plastic tray that can take up to five 8-well slides is placed inside the box. Nylon mesh is from net curtain material obtained from John Lewis Department Store (London).
III. PROCEDURES
A. Preparation of Rat Peritoneal Mast Cells
This procedure has been described in detail previously (Gomperts and Tatham, 1992) and only a brief version is given here.
Solutions
- Percoll at a final density of 1.114 g/ml: Prepare using the following formula:
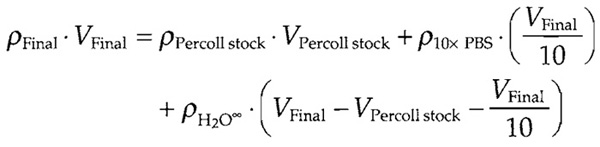 ρ is density (g/ml) and V is volume (ml). Use 10x PBS stock with defined density and highly purified sterile water so that the density may be taken as 1.000g/ml. Prepare 50-100ml of Percoll solution in a sterile environment and store in 2-ml aliquots at -20°C. (may be stored several years). Warm at room temperature, mix thoroughly, and transfer into a pointed bottom tube just before use.
ρ is density (g/ml) and V is volume (ml). Use 10x PBS stock with defined density and highly purified sterile water so that the density may be taken as 1.000g/ml. Prepare 50-100ml of Percoll solution in a sterile environment and store in 2-ml aliquots at -20°C. (may be stored several years). Warm at room temperature, mix thoroughly, and transfer into a pointed bottom tube just before use. - Chloride buffer (CB): Final concentrations 20mM HEPES, 137mM NaCl, 2.7mM KCl, 2mM MgCl2, 1.8mM CaCl2, 5.6mM glucose, and 1mg/ml bovine serum albumin (BSA), pH 7.2. Prepare 10x concentrated stock solution without BSA. To make 500 ml of 10x CB, dissolve 40 g NaCl, 1 g KCl, 23.83 g HEPES, 5 g glucose, 10ml of 1M MgCl2, and 9ml of 1M CaCl2 in distilled water, adjust pH to 7.2 with NaOH, and complete to 500ml. Store in 50-ml aliquots at -20°C. Thaw, mix, dilute, add BSA, and readjust pH to 7.2 before use.
Steps
- Use Sprague-Dawley rats. "Retired breeders" of either sex were used but other strains of various ages are also suitable; older rats provide more cells.
- After peritoneal lavage of rats, pellet the cells present in the washings by centrifugation (5 min at 250g).
- Resuspend cells in ~7ml of CB and filter the suspension through a nylon mesh.
- Overlay the filtered suspension onto a 2-ml cushion of Percoll solution placed in a 10-ml pointed bottom tube.
- Centrifuge (10 min at 250 g, room temperature, slow acceleration). Dense mast cells pellet through the Percoll cushion while contaminating cells (neutrophils, macrophages, red blood cells) remain at the buffer-Percoll interface.
- Remove the buffer, interface cells, and Percoll, resuspend the pellet in ~1 ml CB, and transfer into a clean tube (avoid touching the tube wall where some of the contaminating cells may still adhere).
- Add further ~10ml CB, centrifuge (5 min at 250g), and resuspend pellet in 1.5ml CB. About 1 × 106 cells (>95% purity) are obtained from one rat. This is sufficient for at least five 8-well slides.
SL-O binds to the cells on ice, excess SL-O (and any other additives) is removed by washing with a cold buffer, and cells are then permeabilised by the addition of warm buffer. Permeabilised cells are finally washed to remove freely soluble ions, nucleotides, and proteins before the addition of triggering solutions. A small loss of responsiveness occurs due to the "rundown" during the washing (10-20%) but the advantage is that nucleotides, ions, and proteins leaking out from cells do not come into contact with those applied exogenously. If this is of no concern (e.g., when ATP levels have been depleted by metabolic inhibition), triggers can be added at the time of permeabilisation.
Solutions
- Glutamate buffer (GB): Final concentrations 137mM Kglutamate, 20mM PIPES, 2mM MgCl2, and 1 mg/ml BSA, pH 6.8. Prepare 10x concentrated stock solution without BSA. To make 250ml of 10x GB, dissolve 63.43g glutamic acid, 15.12g PIPES, and 5ml 1M MgCl2 in distilled water, adjust pH to 6.8 with glacial acetic acid, and complete to 250ml. Store 50-ml aliquots at -20°C. Thaw, mix, dilute, add BSA, and readjust pH to 6.8 before use.
- 100mM EGTA stock solution: Store in 5- to 10-ml aliquots at -20°C. To make 100 ml of 100 mM EGTA, dissolve 3.804 g EGTA in 100ml distilled water.
- Glutamate buffer-3 mM EGTA (GBE): Add 300 µl of 100mM EGTA to 10ml GB.
- Streptolysin-O (SL-O): Streptolysin-O from iTEST is supplied as a lyophilized powder in vials of 22IU and must be reduced before use with sodium dithionite. To prepare a working stock at 20IU/ml, dissolve one vial of SL-O in 550µl PBS containing 0.1% BSA and one vial of 20mg sodium dithionite in 550µl PBS containing 0.1% BSA. Mix these two solutions and incubate at 37°C for 1 h. SL-O is now reduced and the solution is ready for use. For storing, divide the reduced SL-O solution into 100-µl aliquots, freeze under liquid nitrogen, and store at -80°C. Just before use, warm and mix and use on the same day. For the "prebind" method, use SL-O at the final concentration of 1.6IU/ml: Add 1.15ml of GBE to 100µl of 20IU SL-O/ml. Thus, one aliquot will provide 1.25ml of working solution, enough for one experiment.
- Clean 8-well slides with distilled water and then ethanol. Leave to dry. Label the slides.
- Pipette 30µl cell suspension in CB per well (i.e., ~20,000 cells per well). Allow 2-4 wells for each condition. Keep 150µl of the cell suspension aside for secretion assay (see solution D3 and step D2).
- Let cells attach for 1 h at room temperature on a plastic tray in a moisture box 1.
1 In some experiments, it is desirable to deplete endogenous ATP from intact cells with metabolic inhibitors. In this case, after step 2, incubate attached intact cells with CB where glucose has been omitted and 10µM antimycin A with 6mM 2-deoxyglucose included. More than 90% depletion of ATP is achieved in 20min at 30°C (Koffer and Churcher, 1993).
- Place the slides on a metal plate on ice.
- Wash the cells once with 30 µl ice-cold GBE.
- Add 30µl ice-cold SL-O (1.6IU/ml GBE) to each well and incubate on ice for 8 min to allow SL-O to bind to the plasma membranes.
- Wash the cells once with 30µl ice-cold GBE to remove unbound SL-O and additives.
- Permeabilise the cells by adding 30 µl warm (37°C) GBE and transferring to a prewarmed moisture box at 37°C for 90 s.
- Chill the cells by placing the slide back on ice.
- Wash once with 30µl GB (without EGTA) to remove freely soluble components.
Solutions
- GB-30µM EGTA: Add 3 µl of 100 mM EGTA to 10ml GB.
- Agent to be tested, dissolved in GB-30µM EGTA. These can include an antibody directed against a specific antigen whose activity is being tested. Dilution of the antibody is 10-20x less than that used for immunostaining.
- 100 mM MgATP: To make 16.5 ml of 100 mM MgATP, add 6.61ml of 0.5M Tris and 1.65ml of 1M MgCl2 to 1 g Na2ATP and add H2O to a final volume of 16.5 ml. Store in 100-µl aliquots at -20°C.
- 10 mM GTPγS: Dilute the 100 mM stock solution with GB just before use.
- Ca2+/EGTA buffers system: These are obtained as described previously (Gomperts and Tatham, 1992; Tatham and Gomperts, 1990) by mixing solutions of Ca:EGTA and EGTA, both at ~100mM and pH 6.8 at specified ratios. Keep 10-ml aliquots of 100mM stock solutions at -20°C. Free Ca2+ concentration is controlled by adding the appropriate Ca2+/EGTA buffer to a final concentration of 3 mM.
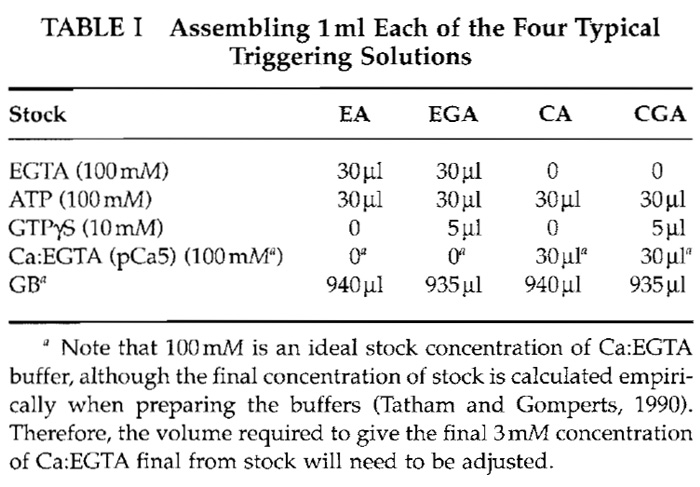
- Triggering solutions: Various combinations of calcium, MgATP, and GTPγS (or GTP) in GB are used for cell stimulation. The following four conditions (final concentrations) are used most frequently: EA (basal condition, 3 mM EGTA-3 mM ATP); EGA (as EA with 50µM GTPγS); CA (3 mM Ca:EGTA/to maintain [Ca2+] at pCa5)-3mM ATP; and CGA (as CA with 50µM GTPγS). Table I gives volumes for making triggering solutions using the aforementioned stock solutions.
 |
Steps
- Immediately after step B9 (permeabilised cells are still on the cold plate), add 15µl of a solution containing the protein or reagent to be tested in GB-30 µMEGTA.
- Incubate on ice (in a moisture box) for 5-30min. The time of incubation depends on the size of the protein/reagent to be introduced into the cells. For example, small proteins such as Rho GTPases require 5-10min but antibodies may require up to 30min to penetrate the SL-O lesions. The time and temperature for action of the agents should be considered.
- Add 15 µl of 2x concentrated triggering solutions containing combinations of calcium/EGTA, MgATP, and GTPγS. (If no pretreatment was required, omit steps 1 and 2 and add 30µl of 1x triggering solutions.)
- Stimulate by transferring to a prewarmed moisture box and incubate for 30min at 37°C. Note that a shorter time (10-15 min) may be sufficient when GTPγS is included in the triggering solution.
- Stop reaction by transferring slides back onto the ice-cold metal plate.
Solutions
- Fluorogenic hexosaminidase substrate: Final concentrations 1mM 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide, 0.1% DMSO, 0.01% Triton X-100, and 200mM citrate, pH 4.5. To make 500ml, mix 189.7mg of the reagent with 0.5 ml DMSO and then add 500ml of 0.2M citrate-0.01% Triton X-100. To make citrate solution, dissolve 21 g of citric acid and 0.5 g of Triton X-100 in H2O, adjust pH to 4.5 using NaOH, and make volume up to 500ml. Using Whatman filter paper No. 1, filter and store in 20- to 50-ml aliquots at -20°C. Hexosaminidase, released from mast cells, hydrolyses the substrate, producing fluorescent 4-methylumbelliferone. Triton reduces surface tension and thus artifacts.
- 0.5M Tris: Dissolve 60.55 g of Tris in water, total volume 1 liter.
- GB
- Solution for assaying total hexosaminidase content: To 150 µl of the original cell suspension (saved at step B2), add 1 ml of 0.2% Triton X-100 in GB and mix to lyse the cells.
- After step C5, remove 15µl (i.e., one-half) of the triggering solutions and transfer into cold transparent 96 V-well plates (on ice).
- Add 100µl of cold GB to each V well using a multichannel pipette.
- Pipette 115 µl of the solution for assaying the total hexosaminidase content into one column of V wells (i.e., 8 × 115 µl).
- Pipette 115µl of GB into one column of V wells (i.e., 8 × 115 µl, to be used for blanks).
- Balance with another 96 V-well plate.
- Centrifuge for 5 min at 250g at 4°C to remove any detached cells.
- Using a multichannel pipette, remove 50µl of the supernatant from each V well and transfer into a black flat-bottom 96-well plate.
- Add 50µl of the fluorogenic hexosaminidase substrate to each well to assay for the release of hexosaminidase.
- Cover with lid and wrap in aluminium foil.
- Incubate 1-2h at 37°C or overnight at room temperature.
- Quench with 100 µl of 0.5 M Tris.
- Read fluorescence using a fluorescence plate reader (excitation and emission filter at 360 and 405 nm, respectively).
- Calculate percentage secretion as follows: % secretion = 100 × (X-B)/(T-B), where X, B, and T are fluorescence values of the sample, average blank, and average total hexosaminidase, respectively.
E. Immunostaining before Fixation
Cells remaining on eight-well slides after removal of the trigger solutions for secretion assays can be stained by fluorescent phalloidin to evaluate the morphology of F-actin (Holt and Koffer, 2000) or be immunostained. Processing of the cells should be done immediately after step D1 and the secretion assay delayed until the cells are in a blocking solution or fixed.
- Stock solution of succinyl-Con A, SCA, 5mg/ml: Dissolve 25 mg in 5 ml GB without BSA. Store 100-µl aliquots at -20°C.
- Glutamate buffer-3 mM EGTA (GBE): Add 300 µl of 100mM EGTA to 10ml GB.
- Blocking solution: 32% goat serum-250µg/ml succinyl-Con A (SCA)-GBE. To make 1 ml, add 320µl goat serum and 50µl SCA (5mg/ml stock) to 630µl GBE.
- Antibody solution: 16% goat serum in GBE. To make 1 ml, add 160 µl goat serum to 840 µl GBE. Higher concentrations of antibodies are usually required if these are introduced into permeabilised cells before fixation than those used for after-fixation staining. Note: After dilution, centrifuge all antibodies for 1 min at ~15,000g to remove any aggregates.
- Fixative: 3% paraformaldehyde in GBE (without BSA)-4% polyethylene glycol (PEG, MW 3200). To make 100ml of fixative, first prepare GB (without BSA)-3mM EGTA-4% (w/v) PEG. Add 3g of paraformaldehyde to ~80ml of GBE-PEG and gently heat and stir in a fume cupboard, adding 10M NaOH in small doses until paraformaldehyde is dissolved and colourless. Adjust pH to 6.8. Filter (in a fume cupboard) using Whatman filter paper No. 1 and store 10-ml aliquots at -20°C.
- Mounting solution: Add 12ml of water to 12g glycerol and 4.8 g Mowiol 4-88 in a conical flask and mix for 2h or overnight at room temperature. Add 24ml 0.2M Tris, pH 8.5, and 400µl of 100mm EGTA and stir further at 50°C (~10min). Centrifuge for 30 min at ~5000g. Discard pellets, stir the supernatants again, and store in 1-ml aliquots at -20°C.
- After step C5 and D1 (i.e., after triggering and removal of aliquots for secretion assay), remove the remaining triggering solutions.
- Wash once in 30µl GBE to remove residual calcium/GTPγS.
- Add 30µl of blocking solution to block unspecific binding.
- Incubate in a moisture box for 30min at room temperature.
- Remove blocking solution and add 30µl of the primary antibody diluted in antibody solution.
- Incubate in a moisture box for 30-60min at room temperature.
- Wash the cells 4x with 30µl GBE.
- Add 30µl of secondary antibody, conjugated to a fluorescent probe and diluted in antibody solution.
- Incubate in a moisture box for 30min at room temperature.
- Wash the cells 4x with 30 µl GBE
- Fix the cells by adding 30µl fixative for 20min at room temperature in a moisture box.
- Wash the cells 4x with 30µl GBE.
- Aspirate wells until dry and add 4µl mounting solution and remove any bubbles.
- Cover eight wells with 18-mm2 coverslips.
To improve access of antibodies into fixed cells, permeabilisation with either lysophosphatidyl choline or digitonin is required. Blocking can be done with a lower concentration of serum when cells are fixed, but succinyl-concanavalin A should still be included because fixation exposes granule matrices that may bind antibody nonspecifically. Including a 5-min wash step with 0.4M NaCl before blocking also reduces nonspecific binding of antibodies to the granule matrices without affecting cellular morphology in fixed cells.
Solutions
- Fixative and mounting solutions as described earlier.
- GBE: Add 300µl of 100mM EGTA to 10ml GB.
- LPC-GBE, 80µg/ml of LPC in GBE: Used for permeabilisation of fixed cells. Stock solution is 40mg of LPC/ml ethanol. Store at -20°C. Warm to ~40°C and mix before use. Dilute 500x, i.e., 2 µl into 1 ml of GBE to obtain the final concentration.
- 0.2 µM digitonin-GBE. Used as an alternative solution for permeabilisation of fixed cells. Digitonin is stored as a 1mM stock solution in DMSO at room temperature. Stock is prepared by dissolving 1.23 mg digitonin/ml in DMSO. Dilute 10µl of stock digitonin in 990 µl GBE and then add 50µl of this 10µM solution to 950µl GBE to prepare the 0.2 µM solution.
- 50mM glycine-GBE: Make 10ml of stock 1M glycine solution by adding 0.75g glycine to 10ml GB (without BSA). Store in 1-ml aliquots at -20°C. Add 50µl of 1M glycine stock to 950µl GBE.
- Blocking solution: 5% goat serum-250µg/ml succinyl-Con A (SCA)-GBE. To make 1 ml, add 50µl goat serum and 50µl stock SCA (5 mg SCA /ml GB without BSA) to 900µl GBE.
- 0.4M NaCl: For 1 ml, add 100µl 4M NaCl to 900 µl GBE.
- Antibody solution: 5% goat serum in GBE. To make 1 ml, add 50µl goat serum to 950µl GBE. After dilution, centrifuge all antibodies for 1 min at ~15,000g to remove any aggregates.
- After step C5 and D1 (i.e., after triggering and removal of aliquots for secretion assay), remove the remaining triggers.
- Fix the cells by adding 30µl fixative for 20min at room temperature in a moisture box.
- Wash the cells 4x with 30µl 50mM glycine-GBE.
- Add 30µl of LPC-GBE (or digitonin-GBE).
- Permeabilise fixed cells for 20min with LPC-GBE or for 5min with digitonin-GBE, both at room temperature.
- Wash the cells 4x with 30µl GBE.
- Remove GBE and add 30µl of 0.4M NaCl in GBE to each well.
- Incubate in a moisture box for 5min at room temperature.
- Wash the cells 4x with 30µl GBE.
- Add 30µl of blocking solution.
- Incubate in a moisture box for 30min at room temperature.
- Remove blocking solution and add 30µl of the primary antibody diluted in antibody solution.
- Incubate in a moisture box for 30-60min at room temperature.
- Wash the cells 4x with 30µl GBE.
- Add 30µl of secondary antibody, conjugated to a fluorescent probe, diluted in antibody solution.
- Incubate in a moisture box for 30min at room temperature.
- Wash the cells 4x with 30µl GBE.
- Aspirate wells until dry and add 4µl mounting solution; remove any bubbles.
- Cover eight wells with 18-mm2 coverslips.
IV. COMMENTS
We have described a method that allows assessment of mast cell secretory function in parallel with studies of cell morphology. An example is shown in Fig. 1. Permeabilised mast cells were stained with anti-β- actin antibody after exposure to the combinations of calcium, ATP, and GTγS described in Table I. Confocal images of equatorial slices are shown together with typical secretory responses shown in parentheses (percentage of released hexosaminidase). The method provides a large potential for investigating the significance of rearrangements of specific molecules to exocytotic function.
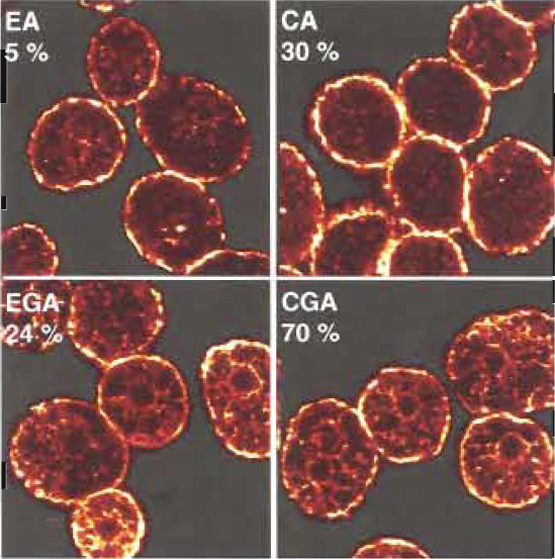 |
| FIGURE 1 Glass-attached mast cells were permeabilised and exposed to the triggers described in Table I: EGTA/ATP (EA), calcium (pCa5)/ATP (CA), EGTA/GTP-γ-S/ATP (EGA), or calcium (pCa 5)/GTPγS/ATP (CGA). After 20min at 37°C, cells were fixed and stained with anti-β-actin monoclonal antibody (clone AC-15) from Sigma (used at 1/200 dilution). The secondary antibody (at 1/50 dilution in GBE) was goat antimouse IgG biotin from Sigma. Cy2 streptavidin (1/50, from Amersham Biosciences, UK) was the tertiary layer. Confocal micrographs of equatorial slices are shown. Numbers indicate the percentages of released hexosaminidase. |
Keep cells in the moisture box as much as possible to avoid evaporation. Maintain permeabilised mast cells at low (25-50µM) concentrations of EGTA before exposure to triggers to avoid spontaneous degranulation. When comparing activities of cells after various pretreatments, keep the time between permeabilisation and triggering standard; the length of the "rundown" period affects secretion. Keep 96-well plates clean, washing them with detergent and plenty of water immediately after use.
Note that when staining is performed before fixation, the nature of the endogenous antigen should be taken into account. Because Soluble proteins will leak out of the permeabilized cells, use this method for molecules known or expected to be tethered either to the membrane or to the actin cytoskeleton. Determination of the extent of leakage of the antigen under various conditions (by immunoblotting of the supernatants) is always helpful.
References
Bhakdi, S., Weller, U., Walev, I., Martin, E., Jonas, D., and Palmer, M. (1993). A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med. Microbiol. Immunol. Berl. 182, 167-175.
Buckingham, L., and Duncan, J. L. (1983). Approximate dimensions of membrane lesions produced by streptolysin S and streptolysin O. Biochim. Biophys. Acta 729, 115-122.
Cockcroft, S., and Gomperts, B. D. (1979). ATP induces nucleotide permeability in rat mast cells. Nature 279, 541-542.
Gomperts, B. D., and Tatham, P. E. R. (1992). Regulated exocytotic secretion from permeabilized cells. Methods Enzymol. 219, 178- 189.
Guo, Z., Turner, C., and Castle, D. (1998). Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell 94, 537-548.
Holt, M., and Koffer, A. (2000). Rho GTPases, secretion and actin dynamics in permeabilised mast cells. Methods Enzymol. 325, 356-369.
Koffer, A., and Gomperts, B. D. (1989). Soluble proteins as modulators of the exocytotic reaction of permeabilised rat mast cells. J. Cell Sci. 94, 585-591.
Larbi, K. Y., and Gomperts, B. D. (1996). Practical considerations regarding the use of streptolysin-O as a permeabilising agent for cells in the investigation of exocytosis. Biosci. Rep. 16, 11-21.
Palmer, M., Harris. R., Freytag, C., Kehoe, M., Tranum Jensen, J., and Bhakdi, S. (1998). Assembly mechanism of the oligomeric streptolysin O pore: The early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 17, 1598-1605.
Tatham, P. E. R., Cusack, N. J., and Gomperts, B. D. (1988). Characterisation of the ATP4- receptor that mediates permeabilisation of rat mast cells. Eur. J. Pharmacol. 147, 13-21.
Tatham, P. E. R., and Gomperts, B. D. (1990). Cell permeabilisation. In "Peptide Hormones: A Practical Approach" (K. Siddle and J. C. Hutton, eds.), pp. 257-269. IRL Press, Oxford.
Tatham, P. E. R., and Lindau, M. (1990). ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J. Gen. Physiol. 95, 459-476.




