Inorganic Ion Transport and the Perception of Light
We shall now follow a cascade that consists of the perception of an environmental signal, the movement of ions across membranes and the resulting changes in transmembrane voltage, and the reaction of the organism to the signal (Fig. 1). Because we know most about the sensory cells of the eye, this system is used as an example, and particularly the role of rhodopsin.Opsin, which loops back and forth across the cell membrane, and rhodopsin are transmembrane proteins. Unwin and Henderson used electron microscopy to determine the structure of bacterial rhodopsin at 7 Å resolution. More recently, the crystal structure of bovine rhodopsin at 2.8 Å resolution was solved by Palczewski and colleagues.
Perception of light is initiated by a photochemical reaction in the retinal cells of the eye, the cis–trans isomerization of 11-cis-retinal (Fig. 5A). 11-Cis-retinal is attached by a Schiff-base linkage between its aldehyde group and the ∈-amino group of a lysine residue in the protein opsin, thus forming rhodopsin. 11-Cis-retinal absorbs light in the visible wavelength region, with an absorption maximum at 500 nm and a molar extinction coefficient of 40,000 cm−1 M−1. The absorption of a single photon of light by 11-cis-retinal, and the subsequent conversion to all-trans-retinal, initiates a series of reactions. These include a conformational change of rhodopsin, and lead to a change in the rate at which sodium ions cross the membrane (see the next paragraph). This results in a change of the voltage across the cell membrane. The same conformational change of rhodopsin, initiated by the absorption of light, leads to the release of all-trans-retinal. The all-transretinal is transformed to all-trans-retinol (Vitamin A) by pigment epithelia cells in the eye. It is a precursor in the synthesis of 11-cis-retinal, which is then transported back to cells containing opsin and reattached to the ∈-amino group of lysine 296 of opsin.
The steps involved in the absorption of light by rhodopsin that lead to changes in the flux of sodium ions across the cell membrane, and to signal transmission, can be summarized as follows. (1) Light is absorbed. (2) Subsequently, the isomerization of retinal results in (3) a conformational change of rhodopsin; leading to (4) the activation of the enzyme cyclic-guanosine monophosphate (cGMP) phosphodiesterase. This results in (5) the hydrolysis of cyclic guanosine monophosphate (cGMP) to 5´ guanosine monophosphate (5´-GMP). (6) cGMP activates a protein that forms Na+-specific channels in the retinal cell membrane and maintains the transmembrane potential Vm at about −40 mV. (7) Lowering the concentration of cGMP in the cell lowers the concentration of open Na+- conducting channels, which to remain open must have cGMP bound to them. (8) The closing of cGMP-activated transmembrane channels decreases the flux rate of Na+ into the cell and, therefore, changes the voltage across the membrane. (9) The changes in Vm result in the release by the retinal cell of chemical signals (neurotransmitters) adjacent to another cell. (10) The neurotransmitters bind to receptors on an adjacent cell that transiently form transmembrane channels, allowing cations or anions, depending on the receptor, to move through the cell membrane.
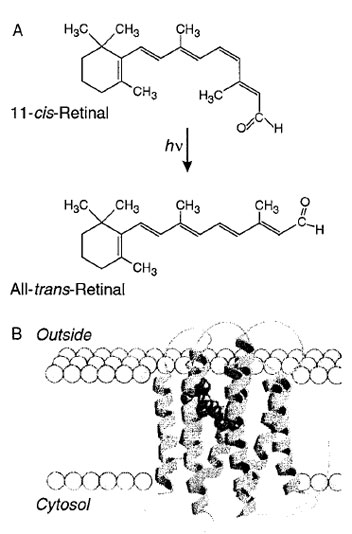 |
| Figure 5 (A) Light-induced transformation of 11-cis-retinal to all-trans-retinal in visual pigments. 11-Cis-retinal is attached through a Schiff-base linkage of lysine 256 in rhodopsin. (B) Rhodopsin has a molecular weight of 40,000. Seven transmembrane helices are embedded in the cell membranes. 11-Cis-retinal lies near the center of the lipid membrane. The structure is based on the three-dimensional reconstruction of electron microscope images by Henderson and Unwin. (Reproduced with permission from Nelson, D. L., and Cox, M. M. p. 460. Figs. 13–22. “Lehninger Principles of Biochemistry” (2000). 3rd edition, Worth Publishers p. 460.) |




