Properties of the Protein (Potassium Channel) That Allows K+ But Not Na+ to Cross the Membrane
Recently, we have learned some of the properties of one channel that plays a central role in rapid signal transmission. The K+ channel from bacteria was crystallized, after a cytoplasmic tail of 33 residues was removed, and MacKinnon and colleagues have determined its structure at a resolution of 3.2 A (Fig. 7). This work represents the first high-resolution, X-ray diffraction study of an ionselective channel. Although the structure was obtained with the bacterial channel, K+ channels with similar se- Figure 7 The selectivity filter of the potassium channel based on the X-ray crystallographic structure determination by MacKinnon and colleagues. The potassium channel is tetrameric with a hole in the middle that forms the ion pore. Each subunit forms two transmembrane helices, the inner and the outer helix. The pore helix and loop regions build up the ion pore in combination with the inner helix. The black spheres in the middle of the channel represent potassium ions. (Reproduced with permission from Branden, C., and Tooze, J. (1999). Fig. 12.11, p. 233. In “Introduction to Protein Structure,” 2nd edition, Garland Publishing, New York.) quences and properties are also present in other organisms. The bacterial K+ channel contains 158 amino acid residues. Four subunits are arranged around a central axis to form the channel. The K+ channel has two transmembrane helices.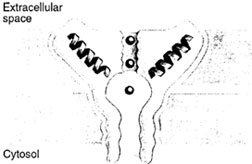 |
| Figure 7 The selectivity filter of the potassium channel based on the X-ray crystallographic structure determination by MacKinnon and colleagues. The potassium channel is tetrameric with a hole in the middle that forms the ion pore . Each subunit forms two transmembrane helices, the inner and the outer helix. The pore helix and loop regions build up the ion pore in combination with the inner helix. The black spheres in the middle of the channel represent potassium ions. (Reproduced with permission from Branden, C., and Tooze, J. (1999). Fig. 12.11, p. 233. In “Introduction to Protein Structure,” 2nd edition, Garland publishing, New York.) |




