Phenolic Antioxidants
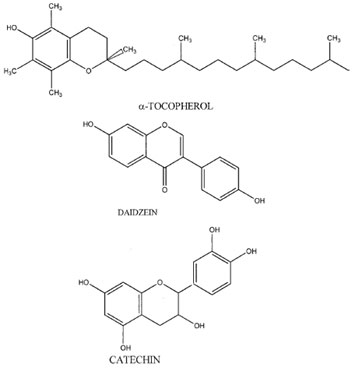
Phenolics are compounds that have a hydroxyl group associated with an aromatic ring structure. There are numerous variations of both natural and synthetic phenolics (see FIGURE 1 Chemical structures of some examples of phenolic antioxidants. Fig. 1 for examples). Natural phenolics are found predominately in the plant kingdom. Vitamin E or α-tocopherol is a plant phenolic required in the diet of humans and other animals. Phenolic compounds primarily inhibit lipid oxidation through their ability to scavenge free radicals and convert the resulting phenolic radicals into a low-energy form that does not further promote oxidation. Chemical properties, including ability of the antioxidant to donate hydrogen to the oxidizing free radical, decrease the energy of the antioxidant radical, and prevent autoxidation of the antioxidant radical into additional free radicals, will influence the antioxidant effectiveness of a free radical scavenger (FRS). In addition, physical partitioning of phenolics will also influence their reactivity. Initially, antioxidant efficiency is dependent on the ability of the FRS to donate a hydrogen to a high energy free radical. As the oxygen–hydrogen bond energy of the FRS decreases, the transfer of the hydrogen to the free radical is more energetically favorable and thus more rapid. The ability of a FRS to donate a hydrogen to a free radical can sometimes be predicted from standard one electron reduction potentials (E°´). If a compound has a reduction potential lower than the reduction potential of a free radical found in a food or biological tissue (e.g., fatty acid based peroxyl radical), it can donate hydrogen to that free radical unless the reaction is kinetically unfeasible. For example, FRS including α-tocopherol (E°´=500 mV), urate (E°´=590 mV), catechol (E°´= 530 mV), and ascorbate (E°´=282mV)all have reduction potentials belowperoxyl radicals (E°´= 1000 mV, a common free radical in lipid oxidation reactions) and therefore can convert the peroxyl radical to a hydroperoxide through hydrogen donation.The efficiency of an antioxidant FRS is also dependent on the energy of the resulting antioxidant radical. If a FRS produces a low energy radical then the likelihood of the FRS radical to promote the oxidation of other molecules is lower and the oxidation reaction rate decreases. Phenolics are effective FRS because phenolic free radicals have low energy due to delocalization of the free radical thoughout the phenolic ring structure. Standard reduction potentials can again be used to help illustrate this point. Radicals on α-tocopherol (E°´=500 mV) and catechol (E°´=530 mV) have lower reduction potentials than polyunsaturated fatty acids (E°´=600 mV), meaning that their radicals do not posses high enough energy to effectively promote the oxidation of unsaturated fatty acids. Effective phenolic anioxidants FRS also produce radicals that do not react rapidly with oxygen to form hydroperoxides that could autoxidize, thus depleting the system of antioxidants. Antioxidant hydroperoxides are also a problem because they can decompose into radicals that could promote oxidation. Thus, if antioxidant hydroperoxides did form, this could result in consumption of the antioxidant with no net decrease in free radicals numbers.
The efficiency of an antioxidant FRS is also dependent on the energy of the resulting antioxidant radical. If a FRS produces a low energy radical then the likelihood of the FRS radical to promote the oxidation of other molecules is lower and the oxidation reaction rate decreases. Phenolics are effective FRS because phenolic free radicals have low energy due to delocalization of the free radical thoughout the phenolic ring structure. Standard reduction potentials can again be used to help illustrate this point. Radicals on α-tocopherol (E°´=500 mV) and catechol (E°´=530 mV) have lower reduction potentials than polyunsaturated fatty acids (E°´=600 mV), meaning that their radicals do not posses high enough energy to effectively promote the oxidation of unsaturated fatty acids. Effective phenolic anioxidants FRS also produce radicals that do not react rapidly with oxygen to form hydroperoxides that could autoxidize, thus depleting the system of antioxidants. Antioxidant hydroperoxides are also a problem because they can decompose into radicals that could promote oxidation. Thus, if antioxidant hydroperoxides did form, this could result in consumption of the antioxidant with no net decrease in free radicals numbers.
Antioxidant radicals may undergo additional reactions that remove radicals from the system, such as reactions with other antioxidant radicals or lipids radicals to form nonradical species. This means that each FRS is capable of inactivating at least of two free radicals, the first being inactivated when the FRS interacts with the initial oxidizing radical, and the second, when the FRS radical interacts with another radical via a termination reaction to form a nonradical product.
Phenolic compounds that act as antioxidants are widespread in the plant kingdom. Plant phenolics can be classified as simple phenolics, phenolic acids, hydroxycinnamic acid derivatives, and flavonoids. In addition to the basic hydroxylated aromatic ring structure of these compounds, plant phenolics are often associated with sugars and organic acids. The consumption of natural plant phenolics have been estimated to be up to 1 g per day. Overall, the presence of phenolics in the diet has been positively associated with the prevention of diseases such as cancer and atherosclerosis. Plant foods high in phenolics include cereals, legumes, and other seeds (e.g., sesame, oats, soybeans, and coffee); red-, purple-, and blue-colored fruits (e.g., grapes, strawberries, and plums); and the leaves of herbs and bushes (e.g., tea, rosemary, and thyme). Many natural phenolics are capable of inhibiting oxidative reactions. However, because phenolics have such a wide array of chemical structures, it is not surprising that antioxidant activities and health benefits vary greatly. Knowledge of antioxidant activity, antioxidant mechanisms, and health benefits of plant phenolics is just beginning to be understood. This section focuses on the best studied of the plant phenolics.
Tocopherols and tocotrienols are a group of phenolic FRS isomers (α, β, δ and γ ; see Fig. 1 for the structure of α-tocopherol) originating in plants and eventually ending up in animal foods via the diet. Interactions between tocopherols and fatty acid peroxyl radicals lead to the formation of fatty acid hydroperoxides and several resonance structures of tocopheroxyl radicals. Tocopheroxyl radicals can interact with other compounds or with each other to form a variety of products. The types and amounts of these products are dependent on oxidation rates, radical species, lipid state (e.g., bulk vs. membrane lipids), and tocopherol concentrations.
Under condition of low oxidation rates in lipid membrane systems, tocopheroxyl radicals primarily convert to tocopherylquinone. Tocopherylquinone can form from the interaction of two tocopheroxyl radicals leading to the formation of tocopherylquinone and the regeneration of tocopherol. Tocopherylquinone can also be regenerated back to tocopherol in the presence of reducing agents (e.g., ascorbic acid). An additional reaction that can occur is the interaction of two tocopheroxyl radicals to form tocopherol dimers.
Tocopherol is found in plant foods especially those high in oil. Soybean, corn, safflower, and cottonseed oil are good sources of α-tocopherol as are whole grains (in particular wheat germ) and tree nuts. All tocopherol isomers are absorbed by humans, but α-tocopherol is preferentially transfered from the liver to lipoproteins, which in turn transports α-tocopherol to tissues. For this reason, α-tocopherol is the isomer most highly correlated with vitamin E activity.
Tea is an important source of dietary antioxidants for humans because it is one of the most common beverages in the world with annual consumption of over 40 liters/ person/year. Phenolics in tea are mainly catechin derivatives, including catechin (Fig. 1), epicatechin, epicatechin gallate, gallocatechin, epigallocatechin gallate, and epigallocatechin. Tea originates from leaves harvested from the bush, Camellia sinensis. Processing of tea leaves involves either blanching to produce green tea or fermenting to produce oolong or black tea. The fermentation process allows polyphenol oxidase enzymes to react with the catechins to form the condensed polyphenols that are responsible for the typical color and flavor of black teas. Green tea leaf extracts contain 38.8% phenolics on a dry weight basis with catechins contributing over 85% of the total phenolics. Condensation of catechins can decrease their solubility; therefore black tea extracts contain less phenolics (24.4%) of which 17% are catechins and 70% are condensed polyphenols (thearubigens). Extraction of phenolics with water from the leaves of rooibos (Aspalathus linearis) resulted in increased antioxidant activity with increasing extraction temperature and time, suggesting that brewing techniques could influence the antioxidant phenolic content of teas. Ingestion of dietary phenolics from tea has been associated with cancer prevention, and absorption of dietary tea phenolics has been reported.
 |
| FIGURE 1 Chemical structures of some examples of phenolic antioxidants. |
Grapes and wines are also significant sources of dietary phenolic antioxidants. Grapes contain a wide variety of phenolics including anthocyanins, flavan-3-ols (catechin), flavonols (quercetin and rutin), and cinnamates (S-glutathionylcaftaric acid). As with many fruits, the majority of grape phenolics are found in the skin, seeds, and stems (collectively termed pomace). During extraction of juice, the pomace is left in contact with the juice for varying times in order to produce products of varying color, with increasing contact time resulting in increased phenolic extraction and, thus, darker color. Therefore, white grape juices and wines have lower phenolics contents (119 mg of gallic acid equivalents/L) than red wines (2057 mg of gallic acid equivalents/L). As would be expected, red grape juice and wines have greater antioxidant capacity due to their higher phenolic content. Both grape juice and wines have been suggested to have positive heath benefits, however, their phenolic compositions are not the same due to differences in juice preparation and changes in phenolic composition that occurs during both fermentation and storage.
The primary phenolics in soybeans are classified as isoflavones. Included among the soybean isoflavones are daidzein (Fig. 1), genistein, and glycitein, and the glycosolated counterparts daidzin, genistin, and glycitin. Unlike the phenolics in grapes and tea, soybean iso flavones are associated with proteins and, therefore, are found in soy flour and not in soybean oil. The concentration of isoflavones in soybeans varies with the environmental conditions under which the beans were grown. In addition, isoflavone concentrations in soy-based foods are altered during food processing operations such as heating and fermentation. Beside whole soybeans, isoflavones are found in soy milk, tempeh, miso, and tofu at concentrations ranging from 294–1625 µg/g product. Genistein and daidzein are absorbed into human plasma from products such as tofu and soy-based beverages. Bioavailability is low, with only 9–21% of the isoflavones being absorbed. Over 90% of the absorbed isoflavones are removed from the plasma within 24 hours.
Herbs and spices often contain high amount of phenolic compounds. For example, rosemary contains carnosic acid, carnosol, and rosmarinic acid. Crude rosemary extracts are a commercially important source of natural phenolic antioxidant additives in foods meats, bulk oils, lipid emulsions, and beverages.




