Form and Function
Form and Funtion
Structures and physiology of protozoan cells are largely the same as those of cells of multicellular organisms. However, because they must conduct all functions of life as individual organisms, and because they show such enormous diversity in form, habitat, and feeding, various protozoan cells have many unique features.
Nucleus and Cytoplasm
As in other eukaryotes, the nucleus is a membrane-bound structure whose interior communicates with the cytoplasm by small pores. Within the nucleus the genetic material (DNA) is borne on chromosomes. Except during cell division, chromosomes are not usually condensed in a form that can be distinguished, although during fixation of the cells for light microscopy, chromosomal material (chromatin) often clumps together irregularly, leaving some areas within the nucleus relatively clear. The appearance is described as vesicular and is characteristic of many protozoan nuclei (Figure 11-1). Condensations of chromatin may be distributed around the periphery of the nucleus or internally in distinct patterns. In some flagellates the chromosomes are visible through interphase as they would appear during prophase of mitosis.
Also within the nucleus, one or more nucleoli are often present. Endosomes are nucleoli that remain as discrete bodies during mitosis; they are characteristic of phytoflagellates, parasitic amebas, and trypanosomes (see Figures 11-1, 11-11, and 11-14).
The macronuclei of ciliates are described as compact or condensed because the chromatin material is more finely dispersed and clear areas cannot be observed with the light microscope (see Figure 11-23).
Cellular organelles like those in cells of multicellular animals can be distinguished in the cytoplasm of many protozoa. These organelles include mitochondria, endoplasmic reticulum, Golgi apparatus, and various vesicles. Chloroplasts, the membrane-bound organelles in which photosynthesis takes place, are found in most phytoflagellates (see Figure 11-12).
Sometimes peripheral and central areas of cytoplasm can be distinguished as ectoplasm and endoplasm (see Figure 11-4). Endoplasm appears more granular and contains the nucleus and cytoplasmic organelles. Ectoplasm appears more transparent (hyaline) by light microscopy, and it bears the bases of the cilia or flagella. Ectoplasm is often more rigid and is in the gel state of a colloid, whereas the more fluid endoplasm is in the sol state.
Characteristics of Protozoan Phyla
Locomotor Organelles
Protozoa move chiefly by cilia and flagella and by pseudopodial movement. These mechanisms are extremely important in the biology of higher animals as well.
Cilia and Flagella
Many small metazoans use cilia not only for locomotion but also to create water currents for their feeding and respiration. Ciliary movement is vital to many species in such functions as handling food, reproduction, excretion, and osmoregulation.
No real morphological distinction exists between cilia and flagella (Figure 11-2), and some investigators have preferred to call them both undulipodia (L. dim. of unda, a wave, + Gr. podos, a foot). However, a cilium propels water parallel to the surface to which the cilium is attached, whereas a flagellum propels water parallel to the main axis of the flagellum. Each flagellum or cilium contains nine pairs of longitudinal microtubules arranged in a circle around a central pair (Figure 11-3), and this is true for all motile flagella and cilia in the animal kingdom, with a few notable exceptions. This “9 + 2” tube of microtubules in a flagellum or cilium is its axoneme; an axoneme is covered by a membrane continuous with the cell membrane covering the rest of the organism. At about the point where an axoneme enters the cell proper, the central pair of microtubules ends at a small plate within the circle of nine pairs (Figure 11-3A). Also at about that point, another microtubule joins each of the nine pairs, so that these form a short tube extending from the base of the flagellum into the cell. The tube consists nine triplets of microtubules and is known as a is the kinetosome (or basal body). Kinetosomes are exactly the same in structure as centrioles that organize mitotic spindles during cell division and Figure 3-22). Centrioles of some flagellates may give rise to kinetosomes, or kinetosomes may function as centrioles. All typical flagella and cilia have a kinetosome at their base, regardless of whether they are borne by a protozoan or metazoan cell.
The current explanation for ciliary and flagellar movement is the sliding microtubule hypothesis. The movement is powered by the release of chemical bond energy in ATP. Two little arms are visible in electron micrographs on each of the pairs of peripheral tubules in the axoneme (level X in Figure 11-3), and these bear the enzyme adenosine triphosphatase (ATPase), which cleaves the ATP. When bond energy in ATP is released, the arms “walk along” one of the filaments in the adjacent pair, causing it to slide relative to the other filament in the pair. Shear resistance, causing the axoneme to bend when the filaments slide past each other, is provided by “spokes” from each doublet to the central pair of fibrils. These spokes are visible in electron micrographs. Direct evidence for the sliding microtubule hypothesis was obtained by attaching tiny gold beads to axonemal microtubules and observing their movement microscopically.
Pseudopodia
Although pseudopodia are the chief means of locomotion in the Sarcodina, they can be formed by a variety of flagellate protozoa, as well as by ameboid cells of many invertebrates. In fact, much defense against disease in the human body depends on ameboid white blood cells, and ameboid cells in many other animals, vertebrate and invertebrate, play similar roles.
In the protozoa, pseudopodia exist in several forms. The most familiar are the lobopodia (Figures 11-4 and 11-5), which are rather large, blunt extensions of the cell body containing both endoplasm and ectoplasm. Some amebas characteristically do not extend individual pseudopodia, but move the whole body with pseudopodial motion; this movement is known as the limax form (for a genus of slugs, Limax). Filopodia are thin extensions, usually branching, and containing only ectoplasm. They are found in members of the sarcodine class Filosea, such as Euglypha (see Figure 11-10B). Reticulopodia (see Figure 11-15) are distinguished from filipodia in that reticulopodia repeatedly rejoin to form a netlike mesh, although some protozoologists believe that the distinction between filipodia and reticulopodia is artificial. Members of the superclass Actinopoda have axopodia (see Figure 11-15), which are long, thin pseudopodia supported by axial rods of microtubules (Figure 11-6). The microtubules are arranged in a definite spiral or geometrical array, depending on the species, and constitute the axoneme of the axopod. Axopodia can be extended or retracted, apparently by addition or removal of microtubular material. Since the tips can adhere to the substrate, the organism can progress by a rolling motion, shortening the axonemes in front and extending those in the rear. Cytoplasm can flow along the axonemes, toward the body on one side and in the reverse direction on the other.
How pseudopodia work has long attracted the interest of zoologists, but only recently have we gained some insight into the phenomenon. When a typical lobopodium begins to form, an extension of ectoplasm called a hyaline cap appears, and endoplasm begins to flow toward and into the hyaline cap (Figures 11-4 and 11-7). The flowing endoplasm contains actin subunits attached to regulatory proteins that prevent actin from polymerizing. As endoplasm flows into the hyaline cap, it fountains out to the periphery. Interaction with lipids in the cell membrane releases the actin subunits from their regulatory proteins and allows them to polymerize into actin microfilaments. The microfilaments become cross-linked to each other by actinbinding protein (ABP) to form a semisolid gel, transforming the ectoplasm into a tube through which the fluid endoplasm flows as the pseudopodium extends. Near the trailing edge of the gel, calcium ions activate an actinsevering protein, releasing microfilaments from the gel and permitting myosin to associate with and pull on these microfilaments. Thus contraction at the trailing edge results in a pressure that forces the fluid endoplasm, along with its now-dissociated actin subunits, back toward the hyaline cap.
Excretion and Osmoregulation
Vacuoles can be seen by light microscopy in the cytoplasm of many protozoa. Some of these vacuoles periodically fill with a fluid substance that is then expelled. Evidence is strong that these contractile vacuoles (see Figures 11-4, 11-12, and 11-23) function principally in osmoregulation. They are more prevalent and fill and empty more frequently in freshwater protozoa than in marine and endosymbiotic species, where the surrounding medium would be more nearly isosmotic (having the same osmotic pressure) to the cytoplasm. Smaller species, which have a greater surface-tovolume ratio, generally have more rapid filling and expulsion rates in their contractile vacuoles. Excretion of metabolic wastes, on the other hand, is almost entirely by diffusion. The main end product of nitrogen metabolism is ammonia, which readily diffuses out of the small bodies of protozoa.
Although it seems clear that contractile vacuoles function to remove excess water that has entered cytoplasm by osmosis, a reasonable explanation for such removal has been elusive. Because no system for pumping water across a membrane is known, it was postulated some years ago that cytoplasmic ions were actively concentrated within vacuoles, water drawn in by osmosis, then the ions were actively resorbed back into the cytoplasm. However, there is no known lipid-bilayer membrane that could retain water against such a concentration gradient. There is some evidence for a more recent hypothesis: Proton pumps on the vacuolar surface and on tubules radiating from it actively transport H+ and cotransport bicarbonate (HCO3 -) (Figure 11-8), which are osmotically active particles. As these particles accumulate within a vacuole, water would be drawn into the vacuole. Fluid within the vacuole would remain isosmotic to the cytoplasm. Then as the vacuole finally joins its membrane to the surface membrane and empties its contents to the outside, it would expel water, H+, and HCO3 -. These ions can be replaced readily by action of carbonic anhydrase on CO2 and H2O. Carbonic anhydrase is present in the cytoplasm of amebas.
Some ciliates, such as Blepharisma, have contractile vacuoles with structure and filling mechanisms apparently similar to those described for amebas. Others, such as Paramecium, have more complex contractile vacuoles. Such vacuoles are located in a specific position beneath the cell membrane, with an “excretory” pore leading to the outside, and surrounded by the ampullae of about six feeder canals (see Figure 11-23). Feeder canals, in turn, are surrounded by fine tubules about 20 nm in diameter, which connect with the canals during filling of the ampullae and at their lower ends connect with the tubular system of endoplasmic reticulum. Ampullae and contractile vacuoles are surrounded by bundles of fibrils, which may function in contraction of these structures. Contraction of the ampullae fills the vacuole. When the vacuole contracts to discharge its contents to the outside, the ampullae become disconnected from the vacuole, so that backflow is prevented. Tubules, ampullae, or vacuoles may be supplied with proton pumps to draw water into their lumens by the mechanism already described.
Nutrition
Protozoa can be categorized broadly into autotrophs (which synthesize their own organic constituents from inorganic substrates) and heterotrophs (which must obtain organic molecules synthesized by other organisms). Another kind of classification, usually applied to heterotrophs, involves those that ingest visible particles of food (phagotrophs, or holozoic feeders) as contrasted with those ingesting food in a soluble form (osmotrophs, or saprozoic feeders). However, reality is not so simple, even among one-celled organisms. Autotrophic protozoa use light energy to synthesize their organic molecules (phototrophs), but they often practice phagotrophy and osmotrophy as well. Even among the heterotrophs, few are exclusively either phagotrophic or osmotrophic. A single order Euglenida (class Phytomastigophorea) contains some forms that are mainly phototrophs, some that are mainly osmotrophs, and some that are mainly phagotrophs. Species of Euglena show considerable variety in nutritional capability. Some species require certain preformed organic molecules, even though they are autotrophs, and some lose their chloroplasts if maintained in darkness, thus becoming permanent osmotrophs.
Holozoic nutrition implies phagocytosis (Figure 11-9), in which an infolding or invagination of the cell membrane surrounds a food particle. As the invagination extends farther into the cell, it is pinched off at the surface. The food particle thus is contained in an intracellular, membrane-bound vesicle, a food vacuole or phagosome. Lysosomes, small vesicles containing digestive enzymes, fuse with the phagosome and pour their contents into it, where digestion begins. As the digested products are absorbed across the vacuolar membrane, the phagosome becomes smaller. Any undigestible material may be released to the outside by exocytosis, the vacuole again fusing with the cell surface membrane. In most ciliates, many flagellates, and many apicomplexans, the site of phagocytosis is a definite mouth structure, the cytostome (Figures 11-9 and 11-23). In amebas, phagocytosis can occur at almost any point by envelopment of the particle with pseudopodia. Particles must be ingested through the opening of the test, or shell, in amebas that have tests. Flagellates may form a temporary cytostome, usually in a characteristic position, or they may have a permanent cytostome with specialized structure. Many ciliates have a characteristic structure for expulsion of waste matter, the cytopyge or cytoproct, found in a characteristic location. In some, the cytopyge also serves as the site for expulsion of the contents of the contractile vacuole.
Saprozoic feeding may be by pinocytosis or by transport of solutes directly across the outer cell membrane. Direct transport across a membrane may be by diffusion, facilitated transport, or active transport. Diffusion is probably of little or no importance in nutrition of protozoa, except possibly in some endosymbiotic species. Some important food molecules, such as glucose and amino acids, may be brought into a cell by facilitated diffusion and active transport.
Reproduction
Sexual phenomena occur widely among protozoa, and sexual processes may precede certain phases of asexual reproduction, but embryonic development does not occur; protozoa do not have embryos. The essential features of sexual processes include a reduction division of the chromosome number to half (diploid number to haploid number), the development of sex cells (gametes) or at least gamete nuclei, and usually a fusion of gamete nuclei.
Fission
The cell multiplication process that produces more individuals in protozoa is called fission. The most common type of fission is binary, in which two essentially identical individuals result (Figure 11-10). When a progeny cell is considerably smaller than the parent and then grows to adult size, the process is called budding. Budding occurs in some ciliates. In multiple fission, division of the cytoplasm (cytokinesis) is preceded by several nuclear divisions, so that a number of individuals are produced almost simultaneously (see Figure 11-20). Multiple fission, or schizogony, is common among the Sporozoea and some Sarcodina. If the multiple fission is preceded by or associated with union of gametes, it is referred to as sporogony.
The foregoing types of division are accompanied by some form of mitosis. However, this mitosis is often somewhat unlike that found in metazoans. For example, the nuclear membrane often persists through mitosis, and the microtubular spindle may be formed within the nuclear membrane. Centrioles have not been observed in nuclear division of ciliates; the nuclear membrane persists in micronuclear mitosis, with the spindle within the nucleus. The macronucleus of ciliates seems simply to elongate, constrict, and divide without any recognizable mitotic phenomena (amitosis).
Sexual Processes
Although all protozoa reproduce asexually, and some are apparently exclusively asexual, the widespread occurrence of sex among protozoa testifies to its importance as a means of genetic recombination. Gamete nuclei, or pronuclei, which fuse in fertilization to restore the diploid number of chromosomes, are usually borne in special gametic cells. When gametes all look alike, they are called isogametes, but most species have two dissimilar types, or anisogametes.
In animals meiosis usually occurs during or just before gamete formation (called gametic meiosis). We see this type of meiosis in Heliozoea, Ciliophora and some flagellates. However, in other flagellates and in Sporozoea, the first divisions after fertilization are meiotic (zygotic meiosis), and all individuals produced asexually (mitotically) in the life cycle up to the next zygote are haploid. Most protozoa that do not reproduce sexually probably are haploid, although demonstration of ploidy is difficult in the absence of meiosis. In some of the Granuloreticulosea (foraminiferans), show an alternation of haploid and diploid generations (intermediary meiosis), a phenomenon widespread among plants.
Fertilization of an individual gamete by another is syngamy, but some sexual phenomena in protozoa do not involve syngamy. Examples are autogamy, in which gametic nuclei arise by meiosis and fuse to form a zygote within the same organism that produced them, and conjugation, in which an exchange of gametic nuclei occurs between paired organisms (conjugants). We will describe conjugation further in the discussion of Paramecium.
Encystment and Excystment
Separated as they are from their external environment only by their delicate external cell membrane, it seems astonishing that protozoa could be so successful in habitats frequently subjected to extremely harsh conditions. Survival under harsh conditions surely is related to the ability to form cysts: dormant forms marked by the possession of resistant external coverings and a more or less complete shutdown of metabolic machinery. Cyst formation is also important to many parasitic forms that must survive a harsh environment between hosts (Figure 11-1). However, some parasites do not form cysts, apparently depending on direct transfer from one host to another. Reproductive phases such as fission, budding, and syngamy may occur in cysts of some species. Encystment has not been found in Paramecium, and it is rare or absent in marine forms.
The conditions stimulating encystment are incompletely understood, although in some cases cyst formation is cyclic, occurring at a certain stage in the life cycle. In most free-living forms, adverse environmental change favors encystment. Such conditions may include food deficiency, desiccation, increased environmental osmotic pressure, decreased oxygen concentration, or change in pH or temperature.
During encystment a number of organelles, such as cilia or flagella, are resorbed, and the Golgi apparatus secretes cyst wall material, which is carried to the surface in vesicles and extruded.
Although the exact stimulus for excystation (escape from cysts) is usually unknown, a return of favorable conditions initiates excystment in those protozoa in which the cysts are a resistant stage. In parasitic forms excystment stimulus may be more specific, requiring conditions similar to those found in the host.
Structures and physiology of protozoan cells are largely the same as those of cells of multicellular organisms. However, because they must conduct all functions of life as individual organisms, and because they show such enormous diversity in form, habitat, and feeding, various protozoan cells have many unique features.
Nucleus and Cytoplasm
As in other eukaryotes, the nucleus is a membrane-bound structure whose interior communicates with the cytoplasm by small pores. Within the nucleus the genetic material (DNA) is borne on chromosomes. Except during cell division, chromosomes are not usually condensed in a form that can be distinguished, although during fixation of the cells for light microscopy, chromosomal material (chromatin) often clumps together irregularly, leaving some areas within the nucleus relatively clear. The appearance is described as vesicular and is characteristic of many protozoan nuclei (Figure 11-1). Condensations of chromatin may be distributed around the periphery of the nucleus or internally in distinct patterns. In some flagellates the chromosomes are visible through interphase as they would appear during prophase of mitosis.
Also within the nucleus, one or more nucleoli are often present. Endosomes are nucleoli that remain as discrete bodies during mitosis; they are characteristic of phytoflagellates, parasitic amebas, and trypanosomes (see Figures 11-1, 11-11, and 11-14).
The macronuclei of ciliates are described as compact or condensed because the chromatin material is more finely dispersed and clear areas cannot be observed with the light microscope (see Figure 11-23).
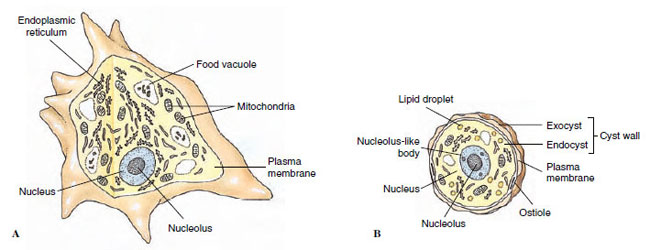 |
| Figure 11-1 Structure of Acanthamoeba palestinensis. A, Active, feeding form. B, Cyst. |
Cellular organelles like those in cells of multicellular animals can be distinguished in the cytoplasm of many protozoa. These organelles include mitochondria, endoplasmic reticulum, Golgi apparatus, and various vesicles. Chloroplasts, the membrane-bound organelles in which photosynthesis takes place, are found in most phytoflagellates (see Figure 11-12).
Sometimes peripheral and central areas of cytoplasm can be distinguished as ectoplasm and endoplasm (see Figure 11-4). Endoplasm appears more granular and contains the nucleus and cytoplasmic organelles. Ectoplasm appears more transparent (hyaline) by light microscopy, and it bears the bases of the cilia or flagella. Ectoplasm is often more rigid and is in the gel state of a colloid, whereas the more fluid endoplasm is in the sol state.
Characteristics of Protozoan Phyla
- Unicellular; some colonial, and some with multicellular stages in their life cycles
- Mostly microscopic, although some are large enough to be seen with the unaided eye
- All symmetries represented in the group; shape variable or constant (oval, spherical, or other)
- No germ layer present
- No organs or tissues, but specialized organelles are found; nucleus single or multiple
- Free-living, mutualism, commensalism, parasitism all represented in the groups
- Locomotion by pseudopodia, flagella, cilia, and direct cell movements; some sessile
- Some provided with a simple endoskeleton or exoskeleton, but most are naked
- Nutrition of all types: autotrophic (manufacturing own nutrients by photosynthesis), heterotrophic (depending on other plants or animals for food), saprozoic (using nutrients dissolved in the surrounding medium)
- Aquatic or terrestrial habitat; free-living or symbiotic mode of life
- Reproduction asexually by fission, budding, and cysts and sexually by conjugation or by syngamy (union of male and female gametes to form a zygote)
Locomotor Organelles
Protozoa move chiefly by cilia and flagella and by pseudopodial movement. These mechanisms are extremely important in the biology of higher animals as well.
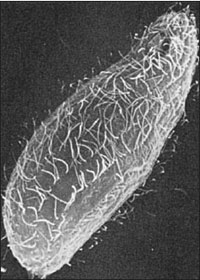 |
| Figure 11-2 Scanning electron micrograph of the free-living ciliate Tetrahymena thermophila howing rows of cilia (x2000). Beating of flagella either pushes or pulls the organism through its medium, while cilia propel the organism by a “rowing” mechanism. Their structure is similar, whether viewed by scanning or transmission electron microscopy. |
Cilia and Flagella
Many small metazoans use cilia not only for locomotion but also to create water currents for their feeding and respiration. Ciliary movement is vital to many species in such functions as handling food, reproduction, excretion, and osmoregulation.
No real morphological distinction exists between cilia and flagella (Figure 11-2), and some investigators have preferred to call them both undulipodia (L. dim. of unda, a wave, + Gr. podos, a foot). However, a cilium propels water parallel to the surface to which the cilium is attached, whereas a flagellum propels water parallel to the main axis of the flagellum. Each flagellum or cilium contains nine pairs of longitudinal microtubules arranged in a circle around a central pair (Figure 11-3), and this is true for all motile flagella and cilia in the animal kingdom, with a few notable exceptions. This “9 + 2” tube of microtubules in a flagellum or cilium is its axoneme; an axoneme is covered by a membrane continuous with the cell membrane covering the rest of the organism. At about the point where an axoneme enters the cell proper, the central pair of microtubules ends at a small plate within the circle of nine pairs (Figure 11-3A). Also at about that point, another microtubule joins each of the nine pairs, so that these form a short tube extending from the base of the flagellum into the cell. The tube consists nine triplets of microtubules and is known as a is the kinetosome (or basal body). Kinetosomes are exactly the same in structure as centrioles that organize mitotic spindles during cell division and Figure 3-22). Centrioles of some flagellates may give rise to kinetosomes, or kinetosomes may function as centrioles. All typical flagella and cilia have a kinetosome at their base, regardless of whether they are borne by a protozoan or metazoan cell.
The current explanation for ciliary and flagellar movement is the sliding microtubule hypothesis. The movement is powered by the release of chemical bond energy in ATP. Two little arms are visible in electron micrographs on each of the pairs of peripheral tubules in the axoneme (level X in Figure 11-3), and these bear the enzyme adenosine triphosphatase (ATPase), which cleaves the ATP. When bond energy in ATP is released, the arms “walk along” one of the filaments in the adjacent pair, causing it to slide relative to the other filament in the pair. Shear resistance, causing the axoneme to bend when the filaments slide past each other, is provided by “spokes” from each doublet to the central pair of fibrils. These spokes are visible in electron micrographs. Direct evidence for the sliding microtubule hypothesis was obtained by attaching tiny gold beads to axonemal microtubules and observing their movement microscopically.
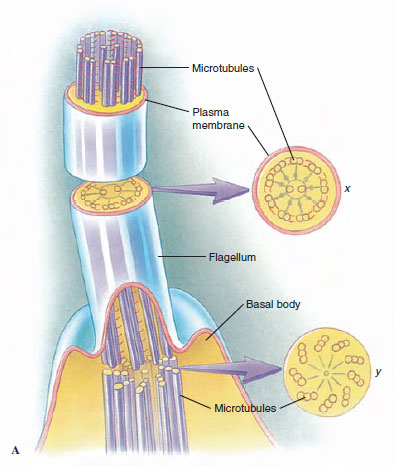 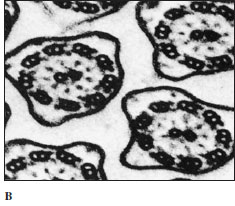 |
| Figure 11-3 A, The axoneme is composed of nine pairs of microtubules plus a central pair, and it is enclosed within the cell membrane. The central pair ends near the level of the cell surface in a basal plate (axosome). The peripheral microtubules continue inward for a short distance to compose two of each of the triplets in the kinetosome (at level y in A). B, Electron micrograph of section through several cilia, corresponding to section x in A. (x133,000) |
Pseudopodia
Although pseudopodia are the chief means of locomotion in the Sarcodina, they can be formed by a variety of flagellate protozoa, as well as by ameboid cells of many invertebrates. In fact, much defense against disease in the human body depends on ameboid white blood cells, and ameboid cells in many other animals, vertebrate and invertebrate, play similar roles.
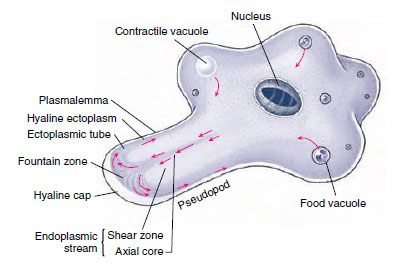 |
| Figure 11-4 Ameba in active locomotion. Arrows indicate the direction of streaming protoplasm. The first sign of a new pseudopodium is thickening of the ectoplasm to form a clear hyaline cap, into which the fluid endoplasm flows. As the endoplasm reaches the forward tip, it fountains out and is converted into ectoplasm, forming a stiff outer tube that lengthens as the forward flow continues. Posteriorly the ectoplasm is converted into fluid endoplasm, replenishing the flow. Substratum is necessary for ameboid movement. |
In the protozoa, pseudopodia exist in several forms. The most familiar are the lobopodia (Figures 11-4 and 11-5), which are rather large, blunt extensions of the cell body containing both endoplasm and ectoplasm. Some amebas characteristically do not extend individual pseudopodia, but move the whole body with pseudopodial motion; this movement is known as the limax form (for a genus of slugs, Limax). Filopodia are thin extensions, usually branching, and containing only ectoplasm. They are found in members of the sarcodine class Filosea, such as Euglypha (see Figure 11-10B). Reticulopodia (see Figure 11-15) are distinguished from filipodia in that reticulopodia repeatedly rejoin to form a netlike mesh, although some protozoologists believe that the distinction between filipodia and reticulopodia is artificial. Members of the superclass Actinopoda have axopodia (see Figure 11-15), which are long, thin pseudopodia supported by axial rods of microtubules (Figure 11-6). The microtubules are arranged in a definite spiral or geometrical array, depending on the species, and constitute the axoneme of the axopod. Axopodia can be extended or retracted, apparently by addition or removal of microtubular material. Since the tips can adhere to the substrate, the organism can progress by a rolling motion, shortening the axonemes in front and extending those in the rear. Cytoplasm can flow along the axonemes, toward the body on one side and in the reverse direction on the other.
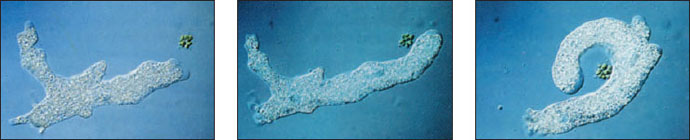 |
| Figure 11-5 Ameboid movement. At left and center, the ameba extends a pseudopodium toward a Pandorina colony. At right, the ameba surrounds the Pandorina before engulfing it by phagocytosis. |
How pseudopodia work has long attracted the interest of zoologists, but only recently have we gained some insight into the phenomenon. When a typical lobopodium begins to form, an extension of ectoplasm called a hyaline cap appears, and endoplasm begins to flow toward and into the hyaline cap (Figures 11-4 and 11-7). The flowing endoplasm contains actin subunits attached to regulatory proteins that prevent actin from polymerizing. As endoplasm flows into the hyaline cap, it fountains out to the periphery. Interaction with lipids in the cell membrane releases the actin subunits from their regulatory proteins and allows them to polymerize into actin microfilaments. The microfilaments become cross-linked to each other by actinbinding protein (ABP) to form a semisolid gel, transforming the ectoplasm into a tube through which the fluid endoplasm flows as the pseudopodium extends. Near the trailing edge of the gel, calcium ions activate an actinsevering protein, releasing microfilaments from the gel and permitting myosin to associate with and pull on these microfilaments. Thus contraction at the trailing edge results in a pressure that forces the fluid endoplasm, along with its now-dissociated actin subunits, back toward the hyaline cap.
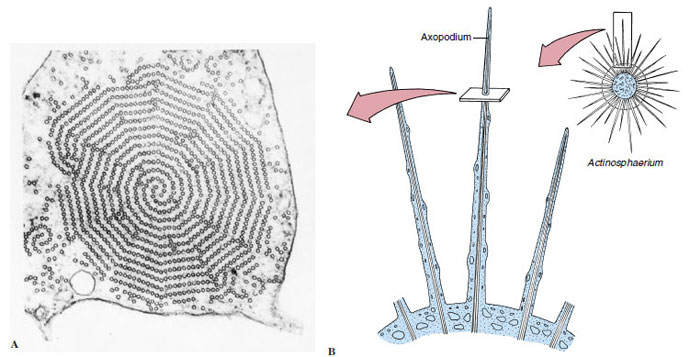 |
| Figure 11-6 A, Electron micrograph of axopodium (from Actinosphaerium nucleofilum) in cross section. B, Diagram of axopodium to show orientation of A. The axoneme of an axopodium is composed of an array of microtubules, which may vary from three to many in number depending on the species. Some species can extend or retract their axopodia quite rapidly. (x99,000) |
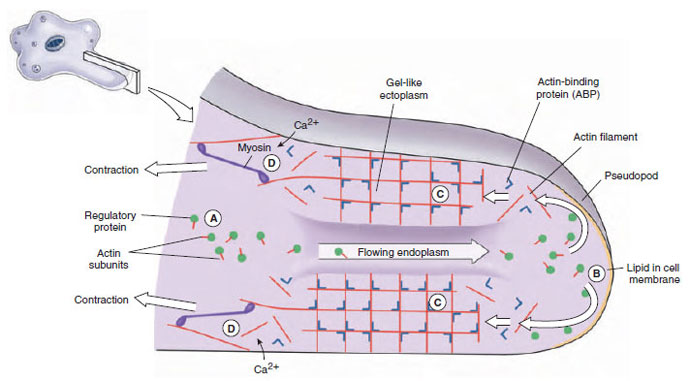 |
| Figure 11-7 Proposed mechanism of pseudopodial movement. In endoplasm, actin subunits are bound to regulatory proteins that keep them from assembling (A). Upon stimulation, hydrostatic force carries the subunits through a weakened gel to the hyaline cap. The actin subunits are freed from the regulatory proteins by lipids in the cell membrane (B). Subunits quickly assemble into filaments and, upon interaction with actin-binding protein (ABP), form gel-like ectoplasm (C). At the trailing edge, calcium ions activate actin-severing proteins, loosening the network enough that myosin molecules can pull on it (D). Subunits pass up through the tube of ectoplasm to be reused. |
Excretion and Osmoregulation
Vacuoles can be seen by light microscopy in the cytoplasm of many protozoa. Some of these vacuoles periodically fill with a fluid substance that is then expelled. Evidence is strong that these contractile vacuoles (see Figures 11-4, 11-12, and 11-23) function principally in osmoregulation. They are more prevalent and fill and empty more frequently in freshwater protozoa than in marine and endosymbiotic species, where the surrounding medium would be more nearly isosmotic (having the same osmotic pressure) to the cytoplasm. Smaller species, which have a greater surface-tovolume ratio, generally have more rapid filling and expulsion rates in their contractile vacuoles. Excretion of metabolic wastes, on the other hand, is almost entirely by diffusion. The main end product of nitrogen metabolism is ammonia, which readily diffuses out of the small bodies of protozoa.
Although it seems clear that contractile vacuoles function to remove excess water that has entered cytoplasm by osmosis, a reasonable explanation for such removal has been elusive. Because no system for pumping water across a membrane is known, it was postulated some years ago that cytoplasmic ions were actively concentrated within vacuoles, water drawn in by osmosis, then the ions were actively resorbed back into the cytoplasm. However, there is no known lipid-bilayer membrane that could retain water against such a concentration gradient. There is some evidence for a more recent hypothesis: Proton pumps on the vacuolar surface and on tubules radiating from it actively transport H+ and cotransport bicarbonate (HCO3 -) (Figure 11-8), which are osmotically active particles. As these particles accumulate within a vacuole, water would be drawn into the vacuole. Fluid within the vacuole would remain isosmotic to the cytoplasm. Then as the vacuole finally joins its membrane to the surface membrane and empties its contents to the outside, it would expel water, H+, and HCO3 -. These ions can be replaced readily by action of carbonic anhydrase on CO2 and H2O. Carbonic anhydrase is present in the cytoplasm of amebas.
Some ciliates, such as Blepharisma, have contractile vacuoles with structure and filling mechanisms apparently similar to those described for amebas. Others, such as Paramecium, have more complex contractile vacuoles. Such vacuoles are located in a specific position beneath the cell membrane, with an “excretory” pore leading to the outside, and surrounded by the ampullae of about six feeder canals (see Figure 11-23). Feeder canals, in turn, are surrounded by fine tubules about 20 nm in diameter, which connect with the canals during filling of the ampullae and at their lower ends connect with the tubular system of endoplasmic reticulum. Ampullae and contractile vacuoles are surrounded by bundles of fibrils, which may function in contraction of these structures. Contraction of the ampullae fills the vacuole. When the vacuole contracts to discharge its contents to the outside, the ampullae become disconnected from the vacuole, so that backflow is prevented. Tubules, ampullae, or vacuoles may be supplied with proton pumps to draw water into their lumens by the mechanism already described.
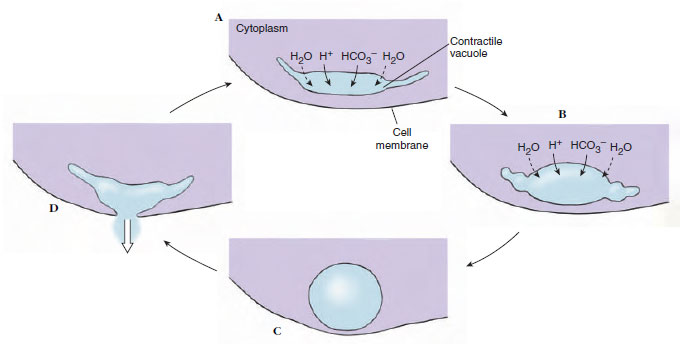 |
| Figure 11-8 Proposed mechanism for operation of contractile vacuoles. A, B, Vacuoles are composed of a system of cisternae and tubules. Proton pumps in their membranes transport H+ and cotransport HCO3− into the vacuoles. Water diffuses in passively to maintain an osmotic pressure equal to that in the cytoplasm. When the vacuole fills C, its membrane fuses with the cell’s surface membrane, expelling water, H+, and HCO3−. D, Protons and bicarbonate ions are replaced readily by action of carbonic anhydrase on carbon dioxide and water. |
Nutrition
Protozoa can be categorized broadly into autotrophs (which synthesize their own organic constituents from inorganic substrates) and heterotrophs (which must obtain organic molecules synthesized by other organisms). Another kind of classification, usually applied to heterotrophs, involves those that ingest visible particles of food (phagotrophs, or holozoic feeders) as contrasted with those ingesting food in a soluble form (osmotrophs, or saprozoic feeders). However, reality is not so simple, even among one-celled organisms. Autotrophic protozoa use light energy to synthesize their organic molecules (phototrophs), but they often practice phagotrophy and osmotrophy as well. Even among the heterotrophs, few are exclusively either phagotrophic or osmotrophic. A single order Euglenida (class Phytomastigophorea) contains some forms that are mainly phototrophs, some that are mainly osmotrophs, and some that are mainly phagotrophs. Species of Euglena show considerable variety in nutritional capability. Some species require certain preformed organic molecules, even though they are autotrophs, and some lose their chloroplasts if maintained in darkness, thus becoming permanent osmotrophs.
Holozoic nutrition implies phagocytosis (Figure 11-9), in which an infolding or invagination of the cell membrane surrounds a food particle. As the invagination extends farther into the cell, it is pinched off at the surface. The food particle thus is contained in an intracellular, membrane-bound vesicle, a food vacuole or phagosome. Lysosomes, small vesicles containing digestive enzymes, fuse with the phagosome and pour their contents into it, where digestion begins. As the digested products are absorbed across the vacuolar membrane, the phagosome becomes smaller. Any undigestible material may be released to the outside by exocytosis, the vacuole again fusing with the cell surface membrane. In most ciliates, many flagellates, and many apicomplexans, the site of phagocytosis is a definite mouth structure, the cytostome (Figures 11-9 and 11-23). In amebas, phagocytosis can occur at almost any point by envelopment of the particle with pseudopodia. Particles must be ingested through the opening of the test, or shell, in amebas that have tests. Flagellates may form a temporary cytostome, usually in a characteristic position, or they may have a permanent cytostome with specialized structure. Many ciliates have a characteristic structure for expulsion of waste matter, the cytopyge or cytoproct, found in a characteristic location. In some, the cytopyge also serves as the site for expulsion of the contents of the contractile vacuole.
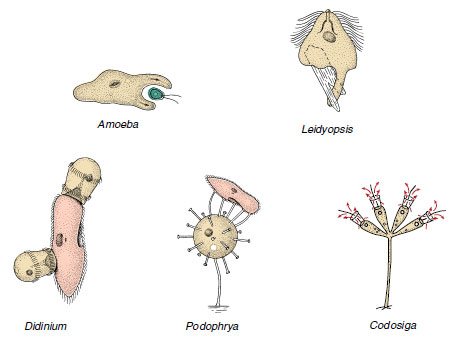 |
| Figure 11-9 Some feeding methods among protozoa. Amoeba surrounds a small flagellate with pseudopodia. Leidyopsis, a flagellate living in the intestine of termites, forms pseudopodia and ingests wood chips. Didinium, a ciliate, feeds only on Paramecium, which it swallows through a temporary cytostome in its anterior end. Sometimes more than one Didinium feed on the same Paramecium. Podophrya is a suctorian ciliophoran. Its tentacles attach to its prey and suck prey cytoplasm into the body of the Podophrya, where it is pinched off to form food vacuoles. Codosiga, a sessile flagellate with a collar of microvilli, feeds on particles suspended in the water drawn through its collar by the beat of its flagellum. Technically, all of these methods are types of phagocytosis. |
Saprozoic feeding may be by pinocytosis or by transport of solutes directly across the outer cell membrane. Direct transport across a membrane may be by diffusion, facilitated transport, or active transport. Diffusion is probably of little or no importance in nutrition of protozoa, except possibly in some endosymbiotic species. Some important food molecules, such as glucose and amino acids, may be brought into a cell by facilitated diffusion and active transport.
Reproduction
Sexual phenomena occur widely among protozoa, and sexual processes may precede certain phases of asexual reproduction, but embryonic development does not occur; protozoa do not have embryos. The essential features of sexual processes include a reduction division of the chromosome number to half (diploid number to haploid number), the development of sex cells (gametes) or at least gamete nuclei, and usually a fusion of gamete nuclei.
Fission
The cell multiplication process that produces more individuals in protozoa is called fission. The most common type of fission is binary, in which two essentially identical individuals result (Figure 11-10). When a progeny cell is considerably smaller than the parent and then grows to adult size, the process is called budding. Budding occurs in some ciliates. In multiple fission, division of the cytoplasm (cytokinesis) is preceded by several nuclear divisions, so that a number of individuals are produced almost simultaneously (see Figure 11-20). Multiple fission, or schizogony, is common among the Sporozoea and some Sarcodina. If the multiple fission is preceded by or associated with union of gametes, it is referred to as sporogony.
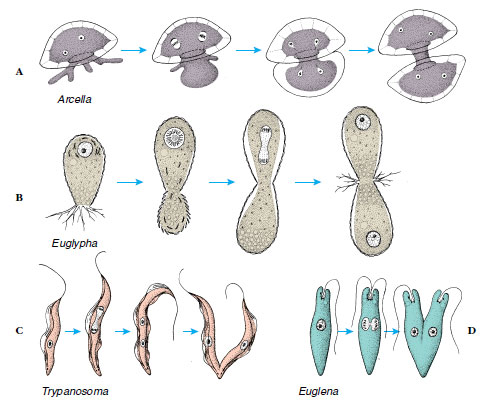 |
| Figure 11-10 Binary fission in some sarcodines and flagellates. A, The two nuclei of Arcella divide as some of its cytoplasm is extruded and begins to secrete a new test for the daughter cell. B, The test of another sarcodine, Euglypha, is constructed of secreted platelets. Secretion of platelets for the daughter cell is begun before cytoplasm begins to move out of the aperture. As these are used to construct the test of the daughter cell, the nucleus divides. C, Trypanosoma has a kinetoplast (part of the mitochondrion) near the kinetosome of its flagellum close to its posterior end in the stage shown. All of these parts must be replicated before the cell divides. D, Division of Euglena. Compare C and D with Figure 11-26, fission in a ciliophoran. |
The foregoing types of division are accompanied by some form of mitosis. However, this mitosis is often somewhat unlike that found in metazoans. For example, the nuclear membrane often persists through mitosis, and the microtubular spindle may be formed within the nuclear membrane. Centrioles have not been observed in nuclear division of ciliates; the nuclear membrane persists in micronuclear mitosis, with the spindle within the nucleus. The macronucleus of ciliates seems simply to elongate, constrict, and divide without any recognizable mitotic phenomena (amitosis).
Sexual Processes
Although all protozoa reproduce asexually, and some are apparently exclusively asexual, the widespread occurrence of sex among protozoa testifies to its importance as a means of genetic recombination. Gamete nuclei, or pronuclei, which fuse in fertilization to restore the diploid number of chromosomes, are usually borne in special gametic cells. When gametes all look alike, they are called isogametes, but most species have two dissimilar types, or anisogametes.
In animals meiosis usually occurs during or just before gamete formation (called gametic meiosis). We see this type of meiosis in Heliozoea, Ciliophora and some flagellates. However, in other flagellates and in Sporozoea, the first divisions after fertilization are meiotic (zygotic meiosis), and all individuals produced asexually (mitotically) in the life cycle up to the next zygote are haploid. Most protozoa that do not reproduce sexually probably are haploid, although demonstration of ploidy is difficult in the absence of meiosis. In some of the Granuloreticulosea (foraminiferans), show an alternation of haploid and diploid generations (intermediary meiosis), a phenomenon widespread among plants.
Fertilization of an individual gamete by another is syngamy, but some sexual phenomena in protozoa do not involve syngamy. Examples are autogamy, in which gametic nuclei arise by meiosis and fuse to form a zygote within the same organism that produced them, and conjugation, in which an exchange of gametic nuclei occurs between paired organisms (conjugants). We will describe conjugation further in the discussion of Paramecium.
Encystment and Excystment
Separated as they are from their external environment only by their delicate external cell membrane, it seems astonishing that protozoa could be so successful in habitats frequently subjected to extremely harsh conditions. Survival under harsh conditions surely is related to the ability to form cysts: dormant forms marked by the possession of resistant external coverings and a more or less complete shutdown of metabolic machinery. Cyst formation is also important to many parasitic forms that must survive a harsh environment between hosts (Figure 11-1). However, some parasites do not form cysts, apparently depending on direct transfer from one host to another. Reproductive phases such as fission, budding, and syngamy may occur in cysts of some species. Encystment has not been found in Paramecium, and it is rare or absent in marine forms.
The conditions stimulating encystment are incompletely understood, although in some cases cyst formation is cyclic, occurring at a certain stage in the life cycle. In most free-living forms, adverse environmental change favors encystment. Such conditions may include food deficiency, desiccation, increased environmental osmotic pressure, decreased oxygen concentration, or change in pH or temperature.
During encystment a number of organelles, such as cilia or flagella, are resorbed, and the Golgi apparatus secretes cyst wall material, which is carried to the surface in vesicles and extruded.
Although the exact stimulus for excystation (escape from cysts) is usually unknown, a return of favorable conditions initiates excystment in those protozoa in which the cysts are a resistant stage. In parasitic forms excystment stimulus may be more specific, requiring conditions similar to those found in the host.




