Phylum Cnidaria
Phylum Cnidaria
Phylum Cnidaria (ny-dar´e-a) (Gr. knide, nettle, + L. aria [pl. suffix], like or connected with) is an interesting group of more than 9000 species. It takes its name from cells called cnidocytes, which contain the stinging organelles (nematocysts) characteristic of the phylum. Nematocysts are formed and used only by cnidarians. Another name for the phylum, Coelenterata (se-len´te-ra´ta) (Gr. koilos, hollow, + enteron, gut, + L. ata [pl. suffix], characterized by), is used less commonly than formerly, and it sometimes now refers to both radiate phyla, since its meaning is equally applicable to both.
Cnidarians are generally regarded as originating close to the basal stock of the metazoan line. They are an ancient group with the longest fossil history of any metazoan, reaching back more than 700 million years. Although their organization has a structural and functional simplicity not found in other metazoans, they form a significant proportion of the biomass in some locations. They are widespread in marine habitats, and there are a few in fresh water. Although they are mostly sessile, or at best, fairly slow moving or slow swimming, they are quite efficient predators of organisms that are much swifter and more complex. The phylum includes some of nature’s strangest and loveliest creatures: branching, plantlike hydroids; flowerlike sea anemones; jellyfishes; and those architects of the ocean floor, horny corals (sea whips, sea fans, and others) and stony corals whose thousands of years of calcareous house-building have produced great reefs and coral islands.
Cnidarians are found most abundantly in shallow marine habitats, especially in warm temperatures and tropical regions. There are no terrestrial species. Colonial hydroids are usually found attached to mollusc shells, rocks, wharves, and other animals in shallow coastal water, but some species are found at great depths. Floating and freeswimming medusae are found in open seas and lakes, often far from shore. Floating colonies such as the Portuguese man-of-war and Velella (L. velum, veil; + ellus, dim. suffix) have floats or sails by which the wind carries them.
Some ctenophores, molluscs, and flatworms eat hydroids bearing nematocysts and use these stinging structures for their own defense. Some other animals feed on cnidarians, but cnidarians rarely serve as food for humans.
Cnidarians sometimes live symbiotically with other animals, often as commensals on the shell or other surface of their host. Certain hydroids (Figure 13-1) and sea anemones commonly live on snail shells inhabited by hermit crabs, providing the crabs some protection from predators. Algae frequently live as mutuals in the tissues of cnidarians, notably in some freshwater hydras and in reef-building corals. The presence of the algae in reef-building corals limits the occurrence of coral reefs to relatively shallow, clear water where there is sufficient light for the photosynthetic requirements of the algae. These kinds of corals are an essential component of coral reefs, and reefs are extremely important habitats in tropical waters. Coral reefs are discussed further later in the section.
Although many cnidarians have little economic importance, reef-building corals are an important exception. Fish and other animals associated with reefs provide substantial amounts of food for humans, and reefs are of economic value as tourist attractions. Precious coral is used for jewelry and ornaments, and coral rock serves for building purposes.
Planktonic medusae may be of some importance as food for fish that are of commercial value; the reverse is also true—young fish fall prey to cnidarians.
Four classes of Cnidaria are commonly recognized: Hydrozoa (most variable class, including hydroids, fire corals, Portuguese man-of-war, and others), Scyphozoa (“true” jellyfishes), Cubozoa (cube jellyfishes), and Anthozoa (largest class, including sea anemones, stony corals, soft corals, and others).
Characteristics of Phylum Cnidaria
Dimorphism and Polymorphism in Cnidarians
One of the most interesting—and sometimes puzzling—aspects of this phylum is the dimorphism and often polymorphism displayed by many of its members. All cnidarian forms fit into one of two morphological types (dimorphism): a polyp, or hydroid form, which is adapted to a sedentary or sessile life, and a medusa, or jellyfish form, which is adapted for a floating or free-swimming existence (Figure 13-2).
Most polyps have tubular bodies with a mouth at one end surrounded by tentacles. The aboral end is usually attached to a substratum by a pedal disc or other device. Polyps may live singly or in colonies. Colonies of some species include morphologically differing individuals (polymorphism) each specialized for a certain function, such as feeding, reproduction, or defense (Figure 13-1).
Medusae are usually free swimming and have bell-shaped or umbrella-shaped bodies and tetramerous symmetry (body parts arranged in fours). The mouth is usually centered on the concave side, and tentacles extend from the rim of the umbrella.
Sea anemones and corals (class Anthozoa) are all polyps: hence, they are not dimorphic. True jellyfishes (class Scyphozoa) have a conspicuous medusoid form, but many have a polypoid larval stage. Colonial hydroids of class Hydrozoa, however, sometimes have life histories that feature both a polyp stage and a free-swimming medusa stage—rather like a Jekyll-and- Hyde existence. A species that has both an attached polyp and a floating medusa within its life history can take advantage of feeding and distribution possibilities of both pelagic (openwater) and benthic (bottom) environments. Many hydrozoans are also polymorphic, with several distinct types of polyps in a colony.
Superficially the polyp and medusa seem very different. But actually each has retained the saclike body plan basic to the phylum (Figure 13-2). A medusa is essentially an unattached polyp with the tubular portion widened and flattened into a bell shape.
Both polyp and medusa possess the three body-wall layers typical of cnidarians, but the jellylike layer of mesoglea is much thicker in a medusa, constituting the bulk of the animal and making it more buoyant. It is because of this mass of mesoglea “jelly” that medusae are commonly called jellyfishes.
Nematocysts: Stinging Organelles
One of the most characteristic structures in the entire cnidarian group is a stinging organelle called a nematocyst (Figure 13-3). Over 20 different types of nematocysts (Figure 13-3) have been described in cnidarians so far; they are important in taxonomic determinations. Nematocysts are tiny capsules composed of material similar to chitin and containing a coiled tubular “thread” or filament, which is a continuation of the narrowed end of the capsule. This end of the capsule is covered by a little lid, or operculum. The inside of the undischarged thread may bear tiny barbs, or spines.
A nematocyst is enclosed in the
cell that has produced it, the cnidocyte (during its development, a cnidocyte
is properly called a cnidoblast).
Except in Anthozoa, cnidocytes are
equipped with a triggerlike cnidocil,
which is a modified cilium. Anthozoan
cnidocytes have a somewhat different
ciliary mechanoreceptor. In some sea
anemones, and perhaps other cnidarians,
small organic molecules from the
prey “tune” the mechanoreceptors,
sensitizing them to the frequency of
vibration caused by the prey swimming.
Tactile stimulation causes the
nematocyst to discharge. Cnidocytes
are borne in invaginations of ectodermal
cells and, in some forms, in gastrodermal
cells, and they are especially
abundant on the tentacles. When a
nematocyst is discharged, its cnidocyte
is absorbed and a new one replaces it.
Not all nematocysts have barbs or
inject poison. Some, for example, do
not penetrate prey but rapidly recoil
like a spring after discharge, grasping
and holding any part of the prey
caught in the coil (Figure 13-4). Adhesive
nematocysts usually do not discharge
in food capture.
The mechanism of nematocyst discharge is remarkable. Evidence indicates that discharge is due to a combination of tensional forces generated during nematocyst formation and to an astonishingly high osmotic pressure within the nematocyst: 140 atmospheres. When stimulated to discharge, the high internal osmotic pressure causes water to rush into the capsule. The operculum opens, and the rapidly increasing hydrostatic pressure within the capsule forces the thread out with great force, turning inside out as it goes. At the everting end of the thread, the barbs flick to the outside like tiny switchblades. This minute but awesome weapon then injects poison when it penetrates prey.
Nematocysts of most cnidarians are not harmful to humans and are a nuisance at worst. However, the stings of a Portuguese man-of-war (see Figure 13-14) and certain jellyfishes are quite painful and sometimes dangerous.
Nerve Net
The nerve net of cnidarians is one of the best examples of a diffuse nervous system in the animal kingdom. This plexus of nerve cells is found both at the base of the epidermis and at the base of the gastrodermis, forming two interconnected nerve nets. Nerve processes (axons) end on other nerve cells at synapses or at junctions with sensory cells or effector organs (nematocysts or epitheliomuscular cells). Nerve impulses are transmitted from one cell to another by release of a neurotransmitter from small vesicles on one side of the synapse or junction. One-way transmission between nerve cells in higher animals is ensured because the vesicles are located on only one side of the synapse. However, cnidarian nerve nets are peculiar in that many of the synapses have vesicles of neurotransmitters on both sides, allowing transmission across the synapse in either direction. Another peculiarity of cnidarian nerves is the absence of any sheathing material (myelin) on the axons.
There is no concentrated grouping of nerve cells to suggest a “central nervous system.” Nerves are grouped, however, in the “ring nerves” of hydrozoan medusae and in marginal sense organs of scyphozoan medusae. In some cnidarians the nerve nets form two or more systems: in Scyphozoa there is a fast conducting system to coordinate swimming movements and a slower one to coordinate movements of tentacles.
Nerve cells of the net have synapses with slender sensory cells that receive external stimuli, and the nerve cells have junctions with epitheliomuscular cells and nematocysts. Together with the contractile fibers of the epitheliomuscular cells, the sensorynerve cell net combination is often termed a neuromuscular system, an important landmark in the evolution of nervous systems. The nerve net arose early in metazoan evolution, and it has never been completely lost phylogenetically. Annelids have it in their digestive systems. In the human digestive system it is represented by nerve plexuses in the musculature. The rhythmical peristaltic movements of the stomach and intestine are coordinated by this counterpart of the cnidarian nerve net.
Class Hydrozoa
The majority of hydrozoa are marine and colonial in form, and a typical life cycle includes both an asexual polyp and a sexual medusa stage. Some, however, such as freshwater hydras, have no medusa stage. Some marine hydroids do not have free medusae (Figure 13-5), whereas some hydrozoans occur only as medusae and have no polyp.
Hydras, although not typical hydrozoans, are widely used as an introduction to Cnidaria because of their small size and ready availability. Combining study of a hydra with that of a representative colonial marine hydroid such as Obelia (Gr. obelias, round cake) gives an excellent idea of the class Hydrozoa.
Hydra: A Freshwater Hydrozoan
Common freshwater hydras (Figure 13-6) are solitary polyps and some of the few cnidarians found in fresh water. Their normal habitat is the underside of aquatic leaves and lily pads in cool, clean fresh water of pools and streams. The hydra family is found throughout the world, with 16 species occurring in North America.
Body Plan: The body of a hydra can extend to a length of 25 to 30 mm or can contract to a tiny, gelatinous mass. It is a cylindrical tube with the aboral end drawn out into a slender stalk, ending in a basal (or pedal) disc for attachment. This basal disc is provided with gland cells to enable a hydra to adhere to a substratum and also to secrete a gas bubble for floating. In the center of the disc there may be an excretory pore. The mouth, located on a conical elevation called the hypostome, is encircled by 6 to 10 hollow tentacles that, like the body, can greatly extend when the animal is hungry.
The mouth opens into the gastrovascular cavity (also called a coelenteron) which communicates with cavities in the tentacles. In some individuals buds may project from the sides, each with a mouth and tentacles like the parent. Testes or ovaries, when present, appear as rounded projections on the surface of the body (Figure 13-6).
Body Wall: The body wall surrounding the gastrovascular cavity consists of an outer epidermis (ectodermal) and an inner gastrodermis (endodermal) with mesoglea between them (Figure 13-3).
Epidermis: The epidermal layer contains epitheliomuscular, interstitial, gland, cnidocyte, and sensory and nerve cells.
Epitheliomuscular cells make up most of the epidermis and serve both for covering and for muscular contraction (Figure 13-7). The bases of most of these cells are extended parallel to the tentacle or body axis and contain myofibrils, thus forming a layer of longitudinal muscle next to the mesoglea. Contraction of these fibrils shortens the body or tentacles.
Interstitial cells are undifferentiated stem cells found among the bases of the epitheliomuscular cells. Differentiation of interstitial cells produces cnidoblasts, sex cells, buds, nerve cells, and others, but generally not epitheliomuscular cells (which reproduce themselves).
Gland cells are tall cells located around the basal disc and mouth, that secrete an adhesive substance for attachment and sometimes a gas bubble for floating.
Cnidocytes containing nematocysts occur throughout the epidermis. Hydras have three functional types of nematocysts: those that penetrate prey and inject poison (penetrants, Figure 13-3), those that recoil and entangle prey (volvents), and those that secrete an adhesive substance used in locomotion and attachment (glutinants).
Sensory cells are scattered among the other epidermal cells, especially near the mouth and tentacles and on the basal disc. The free end of each sensory cell bears a flagellum, which is the sensory receptor for chemical and tactile stimuli. The other end branches into fine processes that synapse with nerve cells.
Nerve cells of the epidermis are generally multipolar (have many processes), although in more highly organized cnidarians the cells may be bipolar (with two processes). Their processes (axons) form synapses with sensory cells and other nerve cells and junctions with epitheliomuscular cells and cnidocytes. There are both oneway (morphologically asymmetrical) and two-way synapses with other nerve cells.
Gastrodermis: The gastrodermis, a layer of cells lining the gastrovascular cavity, contains chiefly large, ciliated, columnar epithelial cells with irregular flat bases. The cells of the gastrodermis include nutritive-muscular, interstitial, and gland cells.
Nutritive-muscular cells are usually tall columnar cells and have laterally extended bases containing myofibrils. The myofibrils run at right angles to the body or tentacle axis and so form a circular muscle layer. However, this muscle layer in hydras is very weak, and longitudinal extension of the body and tentacles is brought about mostly by increasing the volume of water in the gastrovascular cavity. Water is brought in through the mouth by beating of cilia on the nutritivemuscular cells. Thus, water in the gastrovascular cavity serves as a hydrostatic skeleton. The two cilia on the free end of each cell also serve to circulate food and fluids in the digestive cavity. The cells often contain large numbers of food vacuoles. Gastrodermal cells in green hydras (Chlorohydra) (Gr. chloros, green,+ hydra, a mythical nine-headed monster slain by Hercules) bear green algae (zoochlorellae), which give the hydras their color. This existence is probably a case of symbiotic mutualism, since the algae use the respiratory carbon dioxide from the hydra to form organic compounds useful to the host. Algae receive shelter and probably other physiological requirements in return.
Interstitial cells are scattered among the bases of the nutritive cells. They transform into other types of cells when the need arises.
Gland cells in the hypostome and in the column secrete digestive enzymes. Mucous glands surrounding the mouth aid in ingestion.
Nematocysts are not found in the gastrodermis because cnidocytes are lacking in this layer.
Mesoglea: The mesoglea lies between the epidermis and gastrodermis and is attached to both layers. It is gelatinous, or jellylike, and both epidermal and gastrodermal cells send processes into it. It is a continuous layer that extends over both body and tentacles, thickest in the stalk portion and thinnest in the tentacles. This arrangement allows the pedal region to withstand great mechanical strain and gives the tentacles more flexibility. The mesoglea helps to support the body and acts as a type of elastic skeleton.
Locomotion Unlike colonial polyps, which are permanently attached, hydras can move about freely by gliding on a basal disc, aided by mucous secretions. Or using an “inchworm” movement, they bend over and attach tentacles to the substratum. They may even turn end over end or detach themselves and, by forming a gas 1bubble on the basal disc, float to the surface.
Feeding and Digestion Hydras feed on a variety of small crustaceans, insect larvae, and annelid worms. A hydra awaits its prey with tentacles extended (Figure 13-8). The food organism that brushes against its tentacles may find itself harpooned by scores of nematocysts that render it helpless, even though it may be larger than the hydra. The tentacles move toward the mouth, which slowly widens. Well moistened with mucous secretions, the mouth glides over and around the prey, totally engulfing it.
The activator that actually causes the mouth to open is the reduced form of glutathione, which is found to some extent in all living cells. Glutathione escapes from the prey through wounds made by the nematocysts, but only animals releasing enough of the chemical to activate a feeding response are eaten by a hydra. This mechanism explains how a hydra distinguishes between Daphnia, which it relishes, and some other forms that it refuses. If we place glutathione in water containing hydras, each hydra will go through the motions of feeding even though no prey is present.
Inside the gastrovascular cavity, gland cells discharge enzymes on the food. Digestion starts in the gastrovascular cavity (extracellular digestion), but many food particles are drawn by pseudopodia into nutritive-muscular cells of the gastrodermis, where intracellular digestion occurs. Ameboid cells may carry undigested particles to the gastrovascular cavity, where they are eventually expelled with other indigestible matter.
Reproduction Hydras reproduce sexually and asexually. In asexual reproduction, buds appear as outpocketings of the body wall and develop into young hydras that eventually detach from the parent. Most species are dioecious. Temporary gonads (Figure 13-6) usually appear in the autumn, stimulated by lower temperatures and perhaps also by reduced aeration of stagnant waters. Eggs in the ovary usually mature one at a time and are fertilized by sperm shed into the water.
Zygotes undergo holoblastic cleavage to form a hollow blastula. The inner part of the blastula delaminates to form the endoderm (gastrodermis), and the mesoglea is laid down between ectoderm and endoderm. A cyst forms around the embryo before it breaks loose from the parent, enabling it to survive the winter. Young hydras hatch in spring when the weather is favorable.
Hydroid Colonies
Far more representative of class Hydrozoa than hydras are hydroids that have a medusa stage in their life cycle. We often use Obelia in laboratory exercises to illustrate the hydroid type (Figure 13-9).
A typical hydroid has a base, a stalk, and one or more terminal zooids. The base by which the colonial hydroids attach to the substratum is a rootlike stolon, or hydrorhiza, which gives rise to one or more stalks called hydrocauli. The living cellular part of the hydrocaulus is a tubular coenosarc, composed of the three typical cnidarian layers surrounding the coelenteron (gastrovascular cavity). The protective covering of the hydrocaulus is a nonliving chitinous sheath, or perisarc. Attached to the hydrocaulus are individual polyp animals, or zooids. Most zooids are feeding polyps called hydranths, or gastrozooids. They may be tubular, bottle shaped, or vaselike, but all have a terminal mouth and a circle of tentacles. In some forms, such as Obelia, the perisarc continues as a protective cup around the polyp into which it can withdraw for protection (Figure 13-9). In others the polyp is naked (Figure 13-10). In some forms the perisarc is an inconspicuous, thin film.
Hydranths, much like miniature hydras, capture and ingest prey, such as tiny crustaceans, worms, and larvae, thus providing nutrition for the entire colony. After partial extracellular digestion in a hydranth, the digestive broth passes along the common gastrovascular cavity where it is taken up by gastrodermal cells, and intracellular digestion occurs.
Circulation within the gastrovascular cavity is a function of the ciliated gastrodermis but is also aided by rhythmical contractions and pulsations of the body, which occur in hydroids.
Just as hydras reproduce asexually by budding, colonial hydroids bud off new individuals, thus increasing the size of the colony. New feeding polyps arise by budding, and medusa buds also arise on the colony. In Obelia these medusae bud from a reproductive polyp called a gonangium. The young medusae leave the colony as free-swimming individuals that mature and produce gametes (eggs and sperm) (Figure 13-9). In some species medusae remain attached to the colony and shed their gametes there. In other species medusae never develop and gametes are shed by male and female gonophores (Figure 13-10). Embryonation of the zygote results in a ciliated planula larva that swims about for a time. Then it settles down to a substratum to develop into a minute polyp that gives rise, by asexual budding, to the hydroid colony, thus completing the life cycle.
Hydroid medusae are usually smaller than scyphozoan medusae, ranging from 2 to 3 mm to several centimeters in diameter (Figure 13-11). The margin of the bell projects inward as a shelflike velum, which partly closes the open side of the bell and is used in swimming (Figure 13-12). Muscular pulsations that alternately fill and empty the bell propel the animal forward, aboral side first, with a weak “jet propulsion.” Tentacles attached to the bell margin are rich in nematocysts.
The mouth opening at the end of a suspended manubrium leads to a stomach and four radial canals that connect with a ring canal around the margin. This ring canal connects with the hollow tentacles. Thus the gastrovascular cavity is continuous from mouth to tentacles, and gastrodermis lines the entire system. Nutrition is similar to that of hydranths.
The nerve net is usually concentrated into two nerve rings at the base of the velum. The bell margin has a liberal supply of sensory cells. It usually also bears two kinds of specialized sense organs: statocysts, which are small organs of equilibrium (Figure 13-12B), and ocelli, which are light-sensitive organs.
Freshwater Medusae
The freshwater medusa Craspedacusta sowerbyi (Figure 13-13) (order Hydroida) may have evolved from marine ancestors in the Yangtze River of China. Probably introduced with shipments of aquatic plants, this interesting form has now been found in many parts of Europe, all over the United States, and in parts of Canada. Medusae may attain a diameter of 20 mm.
The polyp phase of this animal is tiny (2 mm) and has a very simple form with no perisarc and no tentacles. It occurs in colonies of a few polyps. For a long time its relation to the medusa was not recognized, and thus the polyp was given a name of its own, Microhydra ryderi. On the basis of its relationship to the jellyfish and the law of priority, both polyp and medusa should be called Craspedacusta (N.L. craspedon, velum,+ Gr. kystis, bladder).
The polyp has three methods of asexual reproduction, as shown in Figure 13-13.
Other Hydrozoans
Members of orders Siphonophora and Chondrophora are among the most specialized of the Hydrozoa. They form polymorphic swimming or floating colonies containing several types of modified medusae and polyps.
Physalia (Gr. physallis, bladder), the Portuguese man-of-war (Figure 13-14), is one such colony with a rainbow-hued float of blues and pinks that carries it along the surface waters of tropical seas. Many are blown to shore on the eastern coast of the United States. The long, graceful tentacles, actually zooids, are laden with nematocysts and are capable of inflicting painful stings. The float, called a pneumatophore, is believed to have expanded from the original larval polyp. It contains a sac arising from the body wall and is filled with a gas similar to air. The float acts as a type of nurse-carrier for future generations of individuals that bud from it and hang suspended in the water. Some siphonophores, such as Stephalia and Nectalia, possess swimming bells as well as a float.
There are several types of polyp individuals. Gastrozooids are feeding polyps with a single long tentacle arising from the base of each. Some of these long, stinging tentacles become separated from the feeding polyp and are called dactylozooids, or fishing tentacles. These tentacles sting prey and lift it to the lips of feeding polyps. Among the modified medusoid individuals are the gonophores, which are little more than sacs containing either ovaries or testes.
Other hydrozoans secrete massive calcareous skeletons that resemble true corals (Figure 13-15). They are sometimes called hydrocorals.
Class Scyphozoa
Class Scyphozoa (si-fo-zo´a) (Gr. skyphos, cup) includes most of the larger jellyfishes, or “cup animals.” A few, such as Cyanea (Gr. kyanos, darkblue substance), may attain a bell diameter exceeding 2 m and tentacles 60 to 70 m long (Figure 13-16). Most scyphozoans, however, range from 2 to 40 cm in diameter. Most are found floating in the open sea, some even at depths of 3000 m, but one unusual order is sessile and attaches by a stalk to seaweeds and other objects on the sea bottom (Figure 13-17). Their coloring may range from colorless to striking orange and pink hues.
Bells of different species vary in depth from a shallow saucer shape to a deep helmet or goblet shape. The jelly (mesoglea) layer is unusually thick, giving the bell a fairly firm consistency. Despite the bell’s firmness the jelly is 95% to 96% water. Unlike hydromedusae, this layer in scyphomedusae also contains ameboid cells and fibers. Movement is by rhythmical pulsations of the umbrella. There is no velum as in hydromedusae. Tentacles may be numerous or few, and they may be short as in Aurelia (L. aurum, gold) or long as in Cyanea. Aurelia aurita (Figures 13-18 and 13-19) is a familiar species 7 to 10 cm in diameter, commonly found in the waters off both the east and west coasts of the United States, and widely used for study.
The margin of the umbrella is scalloped,
usually with each indentation
bearing a pair of lappets, and between
them is a sense organ called a rhopalium (tentaculocyst). Aurelia has eight such notches. Some scyphozoans
have 4, others 16. Each rhopalium
is club shaped and contains a hollow
statocyst for equilibrium and one
or two pits lined with sensory epithelium.
In some species the rhopalia also
bear ocelli.
The mouth is centered on the subumbrella side. The manubrium usually forms four frilly oral arms that are used in capturing and ingesting prey.
Tentacles, manubrium, and often the entire body surface are well supplied with nematocysts that can deliver painful stings. However, the primary function of scyphozoan nematocysts is not to attack humans but to paralyze prey animals, which are conveyed to the mouth lobes with the help of other tentacles or by the bending of the umbrella margin.
Aurelia, which has comparatively short tentacles, feeds on small planktonic animals. These are caught in mucus of the umbrella surface, are carried to “food pockets” on the umbrella margin by cilia, and are picked up from the pockets by the oral lobes whose cilia carry the food to the gastrovascular cavity. Cilia in the gastrodermis layer keep a current of water moving to bring food and oxygen into the stomach and expel wastes.
Cassiopeia (L. mythical queen of Ethiopia), the “upside-down jellyfish” common to Florida waters, and Rhizostoma (Gr. rhiza, root,+ stoma, mouth), which can be found in colder temperatures, belong to a group differing from that of Aurelia both in their lack of tentacles on the umbrella margin and in the structure of the oral arms. During development, edges of the oral lobes fold over and fuse, forming canals (arms or brachial canals) that become highly branched. These canals open to the surface at frequent intervals by pores called “mouths”; the original mouth is obliterated in the fusion of the oral lobes. Planktonic organisms caught in the mucus of the frilly oral arms are transported by cilia to the mouths and then up the brachial canals to the gastric cavity. In contrast to the usual swimming habit of medusae, Cassiopeia is usually found lying on its “back” in shallow lagoons. Its umbrella margin contracts about 20 times a minute, creating water currents to bring plankton into contact with the mucus and nematocysts of its oral lobes. Its tissues are abundantly supplied with symbiotic algal cells (zoo-xanthellae). As they lie sunning themselves in shallow water, Cassiopeia are thus reminiscent of large flowers in more ways than one.
Internally, extending out from the stomach of scyphozoans are four gastric pouches in which gastrodermis extends down in little tentacle-like projections called gastric filaments. These filaments are covered with nematocysts to quiet further any prey that may still be struggling. Gastric filaments are lacking in hydromedusae. A complex system of radial canals branches out from the pouches to a ring canal in the margin and forms a part of the gastrovascular cavity.
The nervous system in scyphozoans is a nerve net, with a subumbrella net that controls bell pulsations and another, more diffuse net that controls local reactions such as feeding.
Sexes are separate, with gonads located in the gastric pouches. Fertilization is internal, with sperm being carried by ciliary currents into the gastric pouch of the female. Zygotes may develop in seawater or may be brooded in folds of the oral arms. The ciliated planula larva becomes attached and develops into a scyphistoma, a hydralike form (Figure 13-18). By a process of strobilation the scyphistoma of Aurelia forms a series of saucerlike buds, ephyrae, and is now called a strobila (Figure 13-18). When the ephyrae break loose, they grow into mature jellyfish.
Class Cubozoa
The Cubozoa until recently were considered an order (Cubomedusae) of Scyphozoa. The medusoid is the predominant form (Figure 13-20); the polypoid is inconspicuous and in most cases unknown. Some cubozoan medusae may range up to 25 cm tall, but most are about 2 to 3 cm. In transverse section the bells are almost square. A tentacle or group of tentacles is found at each corner of the square at the umbrella margin. The base of each tentacle is differentiated into a flattened, tough blade called a pedalium (Figure 13-20). Rhopalia are present. The umbrella margin is not scalloped, and the subumbrella edge turns inward to form a velarium. The velarium functions as a velum does in hydrozoan medusae, increasing swimming efficiency, but it differs structurally. Cubomedusae are strong swimmers and voracious predators, feeding mostly on fish. Stings of some species can be fatal to humans.
The complete life cycle is known for only one species, Tripedalia cystophora (L. tri, three, + Gr. pedalion, rudder). The polyp is tiny (1 mm tall), solitary, and sessile. New polyps bud laterally, detach and creep away. Polyps do not produce ephyrae but metamorphose directly into medusae.
Class Anthozoa
Anthozoans, or “flower animals,” are polyps with a flowerlike appearance (Figure 13-21). There is no medusa stage. Anthozoa are all marine and are found in both deep and shallow water and in polar seas as well as tropical seas. They vary greatly in size and may be solitary or colonial. Many forms are supported by skeletons.
The class has three subclasses: Zoantharia (or Hexacorallia), containing sea anemones, hard corals, and others; Ceriantipatharia, containing only tube anemones and thorny corals; and Alcyonaria (or Octocorallia), containing soft and horny corals, such as sea fans, sea pens, sea pansies, and others. Zoantharians and ceriantipatharians have a hexamerous plan (of six or multiples of six) or polymerous symmetry and have simple tubular tentacles arranged in one or more circlets on the oral disc. Alcyonarians are octomerous (built on a plan of eight) and always have eight pinnate (featherlike) tentacles arranged around the margin of the oral disc (Figure 13-22).
The gastrovascular cavity is large and partitioned by septa, or mesenteries, that are inward extensions of the body wall. Where one septum extends into the gastrovascular cavity from the body wall, another extends from the diametrically opposite side; thus, they are said to be coupled. In Zoantharia, the septa are not only coupled, they are also paired (Figure 13-23). The muscular arrangement varies among the different groups, but usually features circular muscles in the body wall and longitudinal and transverse muscles in the septa.
The mesoglea is a mesenchyme containing ameboid cells. A general tendency towards biradial symmetry in the septal arrangement and occurs in the shape of the mouth and pharynx. There are no special organs for respiration or excretion.
Sea Anemones
Sea anemone (order Actiniaria) polyps are larger and heavier than hydrozoan polyps (Figure 13-21). Most range from 5 mm or less to 100 mm in diameter, and from 5 mm to 200 mm long, but some grow much larger. Some sea anemones are quite colorful. Anemones are found in coastal areas all over the world, especially in warmer waters. They attach by means of their pedal discs to shells, rocks, timber, or whatever submerged substrata they can find. Some burrow in mud or sand.
Sea anemones are cylindrical in form with a crown of tentacles arranged in one or more circles around the mouth of the flat oral disc (Figure 13-23). The slit-shaped mouth leads into a pharynx. At one or both ends of the mouth is a ciliated groove called a siphonoglyph, which extends into the pharynx. The siphonoglyph creates a water current directed into the pharynx. Cilia elsewhere on the pharynx direct water outward. Currents thus created carry in oxygen and remove wastes. They also help maintain an internal fluid pressure, providing a hydrostatic skeleton that serves in lieu of a true skeleton as a support for opposing muscles.
The pharynx leads into a large gastrovascular cavity that is divided into six radial chambers by means of six pairs of primary (complete) septa, or mesenteries, extending vertically from the body wall to the pharynx (Figure 13-23). Openings between chambers (septal perforations) in the upper part of the pharyngeal region help in water circulation. Smaller (incomplete) septa partially subdivide the large chambers and provide a means of increasing the surface area of the gastrovascular cavity. The free edge of each incomplete septum forms a type of sinuous cord called a septal filament that is provided with nematocysts and with gland cells for digestion. In some anemones (such as Metridium) the lower ends of the septal filaments are prolonged into acontia threads, also provided with nematocysts and gland cells, that can be protruded through the mouth or through pores in the body wall to help overcome prey or provide defense. The pores also aid in rapid discharge of water from the body when the animal is endangered and contracts to a small size.
Sea anemones are carnivorous, feeding on fish or almost any live (and sometimes dead) animals of suitable size. Some species live on minute forms caught by ciliary currents.
Feeding behavior in many zoantharians is under chemical control. Some respond to reduced glutathione. In certain others two compounds are involved: asparagine, the feeding activator, causes a bending of tentacles toward the mouth; then reduced glutathione induces swallowing of food.
Muscles are well developed in sea anemones, but the arrangement is quite different from that in Hydrozoa. Longitudinal fibers of the epidermis occur only in the tentacles and oral disc of most species. The strong longitudinal muscles of the column are gastrodermal and are located in the septa (Figure 13-23). Gastrodermal circular muscles in the column are well developed.
Most anemones can glide along slowly on their pedal discs. They can expand and stretch their tentacles in search of small vertebrates and invertebrates, which they overpower with tentacles and nematocysts and carry to the mouth. When disturbed, sea anemones contract and withdraw their tentacles and oral discs. Some anemones are able to swim to a limited extent by rhythmical bending movements, which may be a mechanism for escape from enemies such as sea stars and nudibranchs. Stomphia, for example, at the touch of a predatory sea star, will detach its pedal disc and make creeping or swimming movements to escape (Figure 13-24). This escape reaction is elicited not only by the touch of the star but also by exposure to drippings exuded by the star or to crude extracts made from its tissues. The sea drippings contain steroid saponins that are toxic and irritating to most invertebrates. Extracts from nudibranchs also can provoke this reaction in sea anemones.
Anemones form some interesting mutualistic relationships with other organisms. Many species harbor symbiotic algae (zooxanthellae) within their tissues, similar to the hard coralzooxanthellae association (described later in the section), and the anemones profit from the product of algal photosynthesis. Some anemones habitually attach to the shells occupied by certain hermit crabs. The hermit encourages the relationship and, finding its favorite species, which it recognizes by touch, it massages the anemone until it detaches. The hermit crab holds the anemone against its own shell until the anemone is firmly attached. The crab derives some protection against predators by the anemone. The anemone gets free transportation and particles of food dropped by the hermit crab.
Certain damselfishes (anemone fishes) (family Pomacentridae) form associations with large anemones, especially in tropical Indo-Pacific waters (Figure 13-25). An unknown property of the skin mucus of the fish causes the anemone’s nematocysts not to discharge, but if some other fish is so unfortunate as to brush the anemone’s tentacles, it is likely to become a meal. The anemone obviously provides shelter for the anemone fish, and the fish may help ventilate the anemone by its movements, keep the anemone free of sediment, and even lure an unwary victim to seek the same shelter.
Sexes are separate in some sea anemones, and some are hermaphroditic. Monoecious species are protandrous (produce sperm first, then eggs). Gonads are arranged on the margins of the septa, and fertilization takes place externally or in the gastrovascular cavity. The zygote develops into a ciliated larva. Asexual reproduction commonly occurs by pedal laceration or by longitudinal fission, occasionally by transverse fission or by budding. In pedal laceration, small pieces of the pedal disc break off as the animal moves, and each of these regenerates a small anemone.
Zoantharian Corals
The zoantharian corals belong to the order Scleractinia, sometimes known as the true or stony corals. The stony corals might be described as miniature sea anemones that live in calcareous cups they themselves have secreted (Figures 13-26 and 13-27). Like that of the anemones, a coral polyp’s gastrovascular cavity is subdivided by septa arranged in multiples of six (hexamerous) and its hollow tentacles surround the mouth, but there is no siphonoglyph.
Instead of a pedal disc, the epidermis at the base of the column secretes a limy skeletal cup, including sclerosepta, which project up into the polyp between its true septa (Figure 13-27). Living polyps can retract into the safety of their cup when not feeding. Since the skeleton is secreted below the living tissue rather than within it, the calcareous material is an exoskeleton. In many colonial corals, the skeleton may become massive, building up over many years, with the living coral forming a sheet of tissue over the surface (Figure 13-28). The gastrovascular cavities of the polyps are all connected through this sheet of tissue. Three other small orders of Zoantharia are recognized.
Tube Anemones and Thorny Corals
Members of subclass Ceriantipatharia have coupled but unpaired septa. Tube anemones (order Ceriantharia) (Figure 13-29) are solitary and live buried to the level of the oral disc in soft sediments. They occupy tubes constructed of se-creted mucus and threads of nematocyst-like organelles, into which they can withdraw. Thorny or black corals (order Antipatharia) (Figure 13-30) are colonial and attached to a firm substratum. Their skeleton is of a horny material and has thorns. Both of these orders are small in numbers of species and are limited to warmer waters of the sea.
Alcyonarian Corals
Alcyonarians (Octocorallia) have strict octomerous symmetry, with eight pinnate tentacles and eight unpaired, complete septa (Figure 13-22). They are all colonial, and gastrovascular cavities of the polyps communicate through a system of gastrodermal tubes called solenia (Figure 13-31). The solenia run through an extensive mesoglea (coenenchyme) in most alcyonarians, and the surface of the colony is covered by epidermis. The skeleton is secreted in the coenenchyme and consists of limy spicules, fused spicules, or a horny protein, often in combination. Thus the skeletal support of most alcyonarians is an endoskeleton. The variation in pattern among the species of alcyonarians lends great variety to the form of the colonies: from soft corals such as Dendronephthya (Figure 13-32), with their spicules scattered through the coenenchyme, to the tough, axial supports of sea fans and other gorgonian corals (Figure 13-33), to the fused spicules of organ-pipe coral. Renilla (L. ren, kidney, + illa, suffix), the sea pansy, is a colony reminiscent of a pansy flower. Its polyps are embedded in the fleshy upper side, and a short stalk that supports the colony is embedded in the sea floor. Ptilosarcus (Gr. ptilon, feather, + sarkos, flesh), a sea pen, is a member of the same order and may reach a length of 50 cm (Figure 13-22).
The graceful beauty of alcyonarians— in hues of yellow, red, orange, and purple—helps create the “submarine gardens” of the coral reefs.
Coral Reefs
Most students will have seen photographs or movies giving a glimpse of the vibrant color and life found on coral reefs, and some may have been fortunate enough to visit a reef. Coral reefs are among the most productive of all ecosystems, and they have a diversity of life forms rivaled only by tropical rain forests. They are large formations of calcium carbonate (limestone) in shallow tropical seas laid down by living organisms over thousands of years; living plants and animals are confined to the top layer of reefs where they add calcium carbonate to that deposited by their predecessors. The most important organisms that precipitate calcium carbonate from seawater to form reefs are the scleractinian, hermatypic (reef-building) corals (Figure 13-28) and coralline algae. Not only do coralline algae contribute to the total mass of calcium carbonate, but their precipitation of the substance helps to hold the reef together. Some alcyonarians and hydrozoa (especially Millepora [L. mille, thousand, + porus, pore] spp., “fire coral,” Figure 13-15B) contribute in some measure to the calcareous material, and an enormous variety of other organisms contributes small amounts. However, hermatypic (Gr. herma, support, mound, + typos, type) corals seem essential to formation of large reefs, since such reefs do not occur where these corals cannot live.
Hermatypic corals require warmth, light, and the salinity of undiluted seawater. These requirements limit coral reefs to shallow waters between 30 degrees north and 30 degrees south latitude and excludes them from areas with upwelling of cold water or areas near major river outflows with attendant low salinity and high turbidity. These corals require light because they have mutualistic algae (zooxanthellae) living in their tissues. The microscopic zooxanthellae are very important to the corals; their photosynthesis and fixation of carbon dioxide furnish food molecules for their hosts, they recycle phosphorus and nitrogenous waste compounds that otherwise would be lost, and they enhance the ability of the coral to deposit calcium carbonate.
Several types of reefs are commonly recognized. Fringing reefs are close to a landmass with either no lagoon or a narrow lagoon between reef and shore. A barrier reef (Figure 13-34) runs roughly parallel to shore and has a wider and deeper lagoon than does a fringing reef. Atolls are reefs that encircle a lagoon but not an island. These types of reefs typically slope rather steeply into deep water at their seaward edge. Patch or bank reefs occur some distance back from the steep, seaward slope in lagoons of barrier reefs or atolls. The so-called Great Barrier Reef, extending 2027 km long and up to 145 km from shore off the northeast coast of Australia, is actually a complex of reef types.
Fringing, barrier, and atoll reefs all have distinguishable zones that are characterized by different groups of corals and other animals. The side of the reef facing the sea is the reef front or fore reef slope (Figure 13-34). The reef front is more or less parallel to the shore and perpendicular to the predominant direction of wave travel. It slopes downward into deeper water, sometimes gently at first, then precipitously. Characteristic assemblages of scleractinian corals grow deep on the slope, high near the crest, and in intermediate zones. In shallow water or slightly emergent at the top of the reef front is a reef crest. The upper front and the crest bear the greatest force of the waves and must absorb great energy during storms. Pieces of coral and other organisms are broken off at such times and thrown shoreward onto the reef flat, which slopes down into the lagoon. The reef flat thus receives a supply of calcareous material that is eventually broken down into coral sand. The sand is stabilized by the growth of plants such as turtle grass and coralline algae and ultimately becomes cemented into the mass of the reef by precipitation of carbonates. A reef is not an unbroken wall facing the sea but is highly irregular, with grooves, caves, crevices, channels through from the flat to the front, and deep, cup-shaped holes (“blue holes”). Alcyonarians tend to grow in these areas that are more protected from the full force of the waves, as well as on the flat and the deeper areas of the fore reef slope. Many other kinds of organisms inhabit cryptic locations such as caves and crevices.
Enormous numbers of species and individuals of invertebrate groups and fishes populate the reef ecosystem. For example, there are 300 common species of fishes on Caribbean reefs and more than 1200 on the Great Barrier Reef complex of Australia. It is marvelous that such diversity and productivity can be maintained, since reefs are washed by nutrient-poor waves of the open ocean. Although relatively little nutrient enters the ecosystem, little is lost because the interacting organisms are so efficient in recycling. The corals even feed on feces of fish swimming over them!
Phylum Cnidaria (ny-dar´e-a) (Gr. knide, nettle, + L. aria [pl. suffix], like or connected with) is an interesting group of more than 9000 species. It takes its name from cells called cnidocytes, which contain the stinging organelles (nematocysts) characteristic of the phylum. Nematocysts are formed and used only by cnidarians. Another name for the phylum, Coelenterata (se-len´te-ra´ta) (Gr. koilos, hollow, + enteron, gut, + L. ata [pl. suffix], characterized by), is used less commonly than formerly, and it sometimes now refers to both radiate phyla, since its meaning is equally applicable to both.
Cnidarians are generally regarded as originating close to the basal stock of the metazoan line. They are an ancient group with the longest fossil history of any metazoan, reaching back more than 700 million years. Although their organization has a structural and functional simplicity not found in other metazoans, they form a significant proportion of the biomass in some locations. They are widespread in marine habitats, and there are a few in fresh water. Although they are mostly sessile, or at best, fairly slow moving or slow swimming, they are quite efficient predators of organisms that are much swifter and more complex. The phylum includes some of nature’s strangest and loveliest creatures: branching, plantlike hydroids; flowerlike sea anemones; jellyfishes; and those architects of the ocean floor, horny corals (sea whips, sea fans, and others) and stony corals whose thousands of years of calcareous house-building have produced great reefs and coral islands.
Cnidarians are found most abundantly in shallow marine habitats, especially in warm temperatures and tropical regions. There are no terrestrial species. Colonial hydroids are usually found attached to mollusc shells, rocks, wharves, and other animals in shallow coastal water, but some species are found at great depths. Floating and freeswimming medusae are found in open seas and lakes, often far from shore. Floating colonies such as the Portuguese man-of-war and Velella (L. velum, veil; + ellus, dim. suffix) have floats or sails by which the wind carries them.
Some ctenophores, molluscs, and flatworms eat hydroids bearing nematocysts and use these stinging structures for their own defense. Some other animals feed on cnidarians, but cnidarians rarely serve as food for humans.
Cnidarians sometimes live symbiotically with other animals, often as commensals on the shell or other surface of their host. Certain hydroids (Figure 13-1) and sea anemones commonly live on snail shells inhabited by hermit crabs, providing the crabs some protection from predators. Algae frequently live as mutuals in the tissues of cnidarians, notably in some freshwater hydras and in reef-building corals. The presence of the algae in reef-building corals limits the occurrence of coral reefs to relatively shallow, clear water where there is sufficient light for the photosynthetic requirements of the algae. These kinds of corals are an essential component of coral reefs, and reefs are extremely important habitats in tropical waters. Coral reefs are discussed further later in the section.
Although many cnidarians have little economic importance, reef-building corals are an important exception. Fish and other animals associated with reefs provide substantial amounts of food for humans, and reefs are of economic value as tourist attractions. Precious coral is used for jewelry and ornaments, and coral rock serves for building purposes.
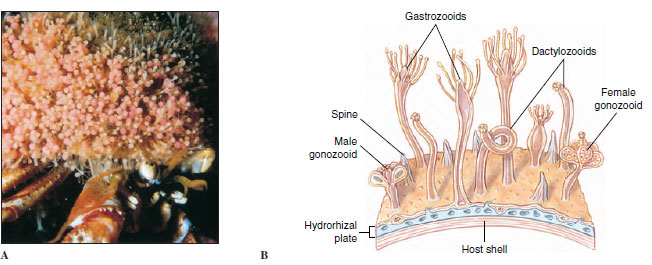 |
| Figure 13-1 A, A hermit crab with its cnidarian mutuals. The shell is blanketed with polyps of the hydrozoan Hydractinia milleri. The crab gets some protection from predation by the cnidarians, and the cnidarians get a free ride and bits of food from their host’s meals. B, Portion of a colony of Hydractinia, showing the types of zooids and the stolon (hydrorhiza) from which they grow. |
Planktonic medusae may be of some importance as food for fish that are of commercial value; the reverse is also true—young fish fall prey to cnidarians.
Four classes of Cnidaria are commonly recognized: Hydrozoa (most variable class, including hydroids, fire corals, Portuguese man-of-war, and others), Scyphozoa (“true” jellyfishes), Cubozoa (cube jellyfishes), and Anthozoa (largest class, including sea anemones, stony corals, soft corals, and others).
Characteristics of Phylum Cnidaria
- Entirely aquatic, some in fresh water but mostly marine
- Radial symmetry or biradial symmetry around a longitudinal axis with oral and aboral ends; no definite head
- Two basic types of individuals: polyps and medusae
- Exoskeleton or endoskeleton of chitinous, calcareous, or protein components in some
- Body with two layers, epidermis and gastrodermis, with mesoglea (diploblastic); mesoglea with cells and connective tissue (ectomesoderm) in some (triploblastic)
- Gastrovascular cavity (often branched or divided with septa) with a single opening that serves as both mouth and anus; extensible tentacles usually encircling the mouth or oral region
- Special stinging cell organelles called nematocysts in either epidermis or gastrodermis or in both; nematocysts abundant on tentacles, where they may form batteries or rings
- Nerve net with symmetrical and asymmetrical synapses; with some sensory organs; diffuse conduction
- Muscular system (epitheliomuscular type) of an outer layer of longitudinal fibers at base of epidermis and an inner one of circular fibers at base of gastrodermis; modifications of this plan in higher cnidarians, such as separate bundles of independent fibers in the mesoglea
- Asexual reproduction by budding (in polyps) or sexual reproduction by gametes (in all medusae and some polyps); sexual forms monoecious or dioecious; planula larva; holoblastic indeterminate cleavage
- No excretory or respiratory system
- No coelomic cavity
Dimorphism and Polymorphism in Cnidarians
One of the most interesting—and sometimes puzzling—aspects of this phylum is the dimorphism and often polymorphism displayed by many of its members. All cnidarian forms fit into one of two morphological types (dimorphism): a polyp, or hydroid form, which is adapted to a sedentary or sessile life, and a medusa, or jellyfish form, which is adapted for a floating or free-swimming existence (Figure 13-2).
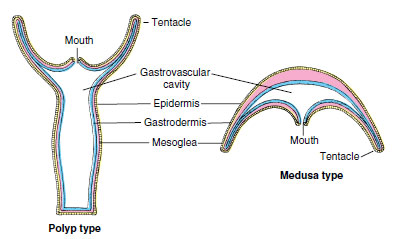 |
| Figure 13-2 Comparison between polyp and medusa types of individuals. |
Most polyps have tubular bodies with a mouth at one end surrounded by tentacles. The aboral end is usually attached to a substratum by a pedal disc or other device. Polyps may live singly or in colonies. Colonies of some species include morphologically differing individuals (polymorphism) each specialized for a certain function, such as feeding, reproduction, or defense (Figure 13-1).
Medusae are usually free swimming and have bell-shaped or umbrella-shaped bodies and tetramerous symmetry (body parts arranged in fours). The mouth is usually centered on the concave side, and tentacles extend from the rim of the umbrella.
Sea anemones and corals (class Anthozoa) are all polyps: hence, they are not dimorphic. True jellyfishes (class Scyphozoa) have a conspicuous medusoid form, but many have a polypoid larval stage. Colonial hydroids of class Hydrozoa, however, sometimes have life histories that feature both a polyp stage and a free-swimming medusa stage—rather like a Jekyll-and- Hyde existence. A species that has both an attached polyp and a floating medusa within its life history can take advantage of feeding and distribution possibilities of both pelagic (openwater) and benthic (bottom) environments. Many hydrozoans are also polymorphic, with several distinct types of polyps in a colony.
Superficially the polyp and medusa seem very different. But actually each has retained the saclike body plan basic to the phylum (Figure 13-2). A medusa is essentially an unattached polyp with the tubular portion widened and flattened into a bell shape.
Both polyp and medusa possess the three body-wall layers typical of cnidarians, but the jellylike layer of mesoglea is much thicker in a medusa, constituting the bulk of the animal and making it more buoyant. It is because of this mass of mesoglea “jelly” that medusae are commonly called jellyfishes.
Nematocysts: Stinging Organelles
One of the most characteristic structures in the entire cnidarian group is a stinging organelle called a nematocyst (Figure 13-3). Over 20 different types of nematocysts (Figure 13-3) have been described in cnidarians so far; they are important in taxonomic determinations. Nematocysts are tiny capsules composed of material similar to chitin and containing a coiled tubular “thread” or filament, which is a continuation of the narrowed end of the capsule. This end of the capsule is covered by a little lid, or operculum. The inside of the undischarged thread may bear tiny barbs, or spines.
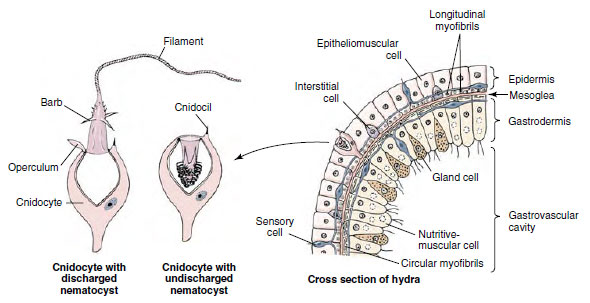 |
| Figure 13-3 At left, structure of a stinging cell. At right, portion of the body wall of a hydra. Cnidocytes, which contain the nematocysts, arise in the epidermis from interstitial cells. |
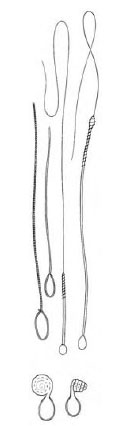 |
| Figure 13-4 Several types of nematocysts shown after discharge. At bottom are two views of a type that does not impale the prey, rather it recoils like a spring, catching any small part of the prey in the path of the recoiling thread. |
The mechanism of nematocyst discharge is remarkable. Evidence indicates that discharge is due to a combination of tensional forces generated during nematocyst formation and to an astonishingly high osmotic pressure within the nematocyst: 140 atmospheres. When stimulated to discharge, the high internal osmotic pressure causes water to rush into the capsule. The operculum opens, and the rapidly increasing hydrostatic pressure within the capsule forces the thread out with great force, turning inside out as it goes. At the everting end of the thread, the barbs flick to the outside like tiny switchblades. This minute but awesome weapon then injects poison when it penetrates prey.
Nematocysts of most cnidarians are not harmful to humans and are a nuisance at worst. However, the stings of a Portuguese man-of-war (see Figure 13-14) and certain jellyfishes are quite painful and sometimes dangerous.
Nerve Net
The nerve net of cnidarians is one of the best examples of a diffuse nervous system in the animal kingdom. This plexus of nerve cells is found both at the base of the epidermis and at the base of the gastrodermis, forming two interconnected nerve nets. Nerve processes (axons) end on other nerve cells at synapses or at junctions with sensory cells or effector organs (nematocysts or epitheliomuscular cells). Nerve impulses are transmitted from one cell to another by release of a neurotransmitter from small vesicles on one side of the synapse or junction. One-way transmission between nerve cells in higher animals is ensured because the vesicles are located on only one side of the synapse. However, cnidarian nerve nets are peculiar in that many of the synapses have vesicles of neurotransmitters on both sides, allowing transmission across the synapse in either direction. Another peculiarity of cnidarian nerves is the absence of any sheathing material (myelin) on the axons.
There is no concentrated grouping of nerve cells to suggest a “central nervous system.” Nerves are grouped, however, in the “ring nerves” of hydrozoan medusae and in marginal sense organs of scyphozoan medusae. In some cnidarians the nerve nets form two or more systems: in Scyphozoa there is a fast conducting system to coordinate swimming movements and a slower one to coordinate movements of tentacles.
Nerve cells of the net have synapses with slender sensory cells that receive external stimuli, and the nerve cells have junctions with epitheliomuscular cells and nematocysts. Together with the contractile fibers of the epitheliomuscular cells, the sensorynerve cell net combination is often termed a neuromuscular system, an important landmark in the evolution of nervous systems. The nerve net arose early in metazoan evolution, and it has never been completely lost phylogenetically. Annelids have it in their digestive systems. In the human digestive system it is represented by nerve plexuses in the musculature. The rhythmical peristaltic movements of the stomach and intestine are coordinated by this counterpart of the cnidarian nerve net.
 |
| Figure 13-5 In some hydroids, such as this Tubularia crocea, medusae are reduced to gonadal tissue and do not detach. These reduced medusae are known as gonophores. |
Class Hydrozoa
The majority of hydrozoa are marine and colonial in form, and a typical life cycle includes both an asexual polyp and a sexual medusa stage. Some, however, such as freshwater hydras, have no medusa stage. Some marine hydroids do not have free medusae (Figure 13-5), whereas some hydrozoans occur only as medusae and have no polyp.
Hydras, although not typical hydrozoans, are widely used as an introduction to Cnidaria because of their small size and ready availability. Combining study of a hydra with that of a representative colonial marine hydroid such as Obelia (Gr. obelias, round cake) gives an excellent idea of the class Hydrozoa.
Hydra: A Freshwater Hydrozoan
Common freshwater hydras (Figure 13-6) are solitary polyps and some of the few cnidarians found in fresh water. Their normal habitat is the underside of aquatic leaves and lily pads in cool, clean fresh water of pools and streams. The hydra family is found throughout the world, with 16 species occurring in North America.
Body Plan: The body of a hydra can extend to a length of 25 to 30 mm or can contract to a tiny, gelatinous mass. It is a cylindrical tube with the aboral end drawn out into a slender stalk, ending in a basal (or pedal) disc for attachment. This basal disc is provided with gland cells to enable a hydra to adhere to a substratum and also to secrete a gas bubble for floating. In the center of the disc there may be an excretory pore. The mouth, located on a conical elevation called the hypostome, is encircled by 6 to 10 hollow tentacles that, like the body, can greatly extend when the animal is hungry.
The mouth opens into the gastrovascular cavity (also called a coelenteron) which communicates with cavities in the tentacles. In some individuals buds may project from the sides, each with a mouth and tentacles like the parent. Testes or ovaries, when present, appear as rounded projections on the surface of the body (Figure 13-6).
 |
| Figure 13-6 Hydra with developing bud and ovary. |
Body Wall: The body wall surrounding the gastrovascular cavity consists of an outer epidermis (ectodermal) and an inner gastrodermis (endodermal) with mesoglea between them (Figure 13-3).
Epidermis: The epidermal layer contains epitheliomuscular, interstitial, gland, cnidocyte, and sensory and nerve cells.
Epitheliomuscular cells make up most of the epidermis and serve both for covering and for muscular contraction (Figure 13-7). The bases of most of these cells are extended parallel to the tentacle or body axis and contain myofibrils, thus forming a layer of longitudinal muscle next to the mesoglea. Contraction of these fibrils shortens the body or tentacles.
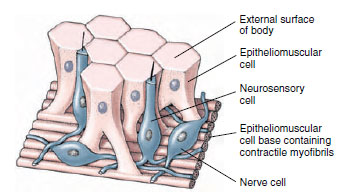 |
| Figure 13-7 Epitheliomuscular and nerve cells in hydra. |
Interstitial cells are undifferentiated stem cells found among the bases of the epitheliomuscular cells. Differentiation of interstitial cells produces cnidoblasts, sex cells, buds, nerve cells, and others, but generally not epitheliomuscular cells (which reproduce themselves).
Gland cells are tall cells located around the basal disc and mouth, that secrete an adhesive substance for attachment and sometimes a gas bubble for floating.
Cnidocytes containing nematocysts occur throughout the epidermis. Hydras have three functional types of nematocysts: those that penetrate prey and inject poison (penetrants, Figure 13-3), those that recoil and entangle prey (volvents), and those that secrete an adhesive substance used in locomotion and attachment (glutinants).
Sensory cells are scattered among the other epidermal cells, especially near the mouth and tentacles and on the basal disc. The free end of each sensory cell bears a flagellum, which is the sensory receptor for chemical and tactile stimuli. The other end branches into fine processes that synapse with nerve cells.
Nerve cells of the epidermis are generally multipolar (have many processes), although in more highly organized cnidarians the cells may be bipolar (with two processes). Their processes (axons) form synapses with sensory cells and other nerve cells and junctions with epitheliomuscular cells and cnidocytes. There are both oneway (morphologically asymmetrical) and two-way synapses with other nerve cells.
Gastrodermis: The gastrodermis, a layer of cells lining the gastrovascular cavity, contains chiefly large, ciliated, columnar epithelial cells with irregular flat bases. The cells of the gastrodermis include nutritive-muscular, interstitial, and gland cells.
Nutritive-muscular cells are usually tall columnar cells and have laterally extended bases containing myofibrils. The myofibrils run at right angles to the body or tentacle axis and so form a circular muscle layer. However, this muscle layer in hydras is very weak, and longitudinal extension of the body and tentacles is brought about mostly by increasing the volume of water in the gastrovascular cavity. Water is brought in through the mouth by beating of cilia on the nutritivemuscular cells. Thus, water in the gastrovascular cavity serves as a hydrostatic skeleton. The two cilia on the free end of each cell also serve to circulate food and fluids in the digestive cavity. The cells often contain large numbers of food vacuoles. Gastrodermal cells in green hydras (Chlorohydra) (Gr. chloros, green,+ hydra, a mythical nine-headed monster slain by Hercules) bear green algae (zoochlorellae), which give the hydras their color. This existence is probably a case of symbiotic mutualism, since the algae use the respiratory carbon dioxide from the hydra to form organic compounds useful to the host. Algae receive shelter and probably other physiological requirements in return.
Interstitial cells are scattered among the bases of the nutritive cells. They transform into other types of cells when the need arises.
Gland cells in the hypostome and in the column secrete digestive enzymes. Mucous glands surrounding the mouth aid in ingestion.
Nematocysts are not found in the gastrodermis because cnidocytes are lacking in this layer.
Mesoglea: The mesoglea lies between the epidermis and gastrodermis and is attached to both layers. It is gelatinous, or jellylike, and both epidermal and gastrodermal cells send processes into it. It is a continuous layer that extends over both body and tentacles, thickest in the stalk portion and thinnest in the tentacles. This arrangement allows the pedal region to withstand great mechanical strain and gives the tentacles more flexibility. The mesoglea helps to support the body and acts as a type of elastic skeleton.
Locomotion Unlike colonial polyps, which are permanently attached, hydras can move about freely by gliding on a basal disc, aided by mucous secretions. Or using an “inchworm” movement, they bend over and attach tentacles to the substratum. They may even turn end over end or detach themselves and, by forming a gas 1bubble on the basal disc, float to the surface.
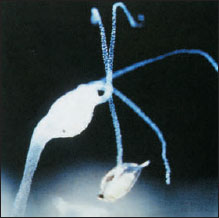 |
| Figure 13-8 Hydra catches an unwary water flea with the nematocysts of its tentacles. This hydra already contains one water flea eaten previously. |
Feeding and Digestion Hydras feed on a variety of small crustaceans, insect larvae, and annelid worms. A hydra awaits its prey with tentacles extended (Figure 13-8). The food organism that brushes against its tentacles may find itself harpooned by scores of nematocysts that render it helpless, even though it may be larger than the hydra. The tentacles move toward the mouth, which slowly widens. Well moistened with mucous secretions, the mouth glides over and around the prey, totally engulfing it.
The activator that actually causes the mouth to open is the reduced form of glutathione, which is found to some extent in all living cells. Glutathione escapes from the prey through wounds made by the nematocysts, but only animals releasing enough of the chemical to activate a feeding response are eaten by a hydra. This mechanism explains how a hydra distinguishes between Daphnia, which it relishes, and some other forms that it refuses. If we place glutathione in water containing hydras, each hydra will go through the motions of feeding even though no prey is present.
Inside the gastrovascular cavity, gland cells discharge enzymes on the food. Digestion starts in the gastrovascular cavity (extracellular digestion), but many food particles are drawn by pseudopodia into nutritive-muscular cells of the gastrodermis, where intracellular digestion occurs. Ameboid cells may carry undigested particles to the gastrovascular cavity, where they are eventually expelled with other indigestible matter.
Reproduction Hydras reproduce sexually and asexually. In asexual reproduction, buds appear as outpocketings of the body wall and develop into young hydras that eventually detach from the parent. Most species are dioecious. Temporary gonads (Figure 13-6) usually appear in the autumn, stimulated by lower temperatures and perhaps also by reduced aeration of stagnant waters. Eggs in the ovary usually mature one at a time and are fertilized by sperm shed into the water.
Zygotes undergo holoblastic cleavage to form a hollow blastula. The inner part of the blastula delaminates to form the endoderm (gastrodermis), and the mesoglea is laid down between ectoderm and endoderm. A cyst forms around the embryo before it breaks loose from the parent, enabling it to survive the winter. Young hydras hatch in spring when the weather is favorable.
Hydroid Colonies
Far more representative of class Hydrozoa than hydras are hydroids that have a medusa stage in their life cycle. We often use Obelia in laboratory exercises to illustrate the hydroid type (Figure 13-9).
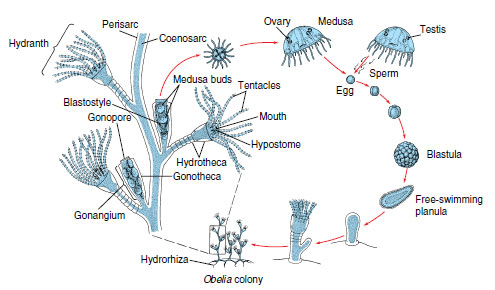 |
| Figure 13-9 Life cycle of Obelia, showing alternation of polyp (asexual) and medusa (sexual) stages. Obelia is a calyptoblastic hydroid; its polyps as well as its stems are protected by continuations of the perisarc. Contrast with Eudendrium (Figure 13-10). |
A typical hydroid has a base, a stalk, and one or more terminal zooids. The base by which the colonial hydroids attach to the substratum is a rootlike stolon, or hydrorhiza, which gives rise to one or more stalks called hydrocauli. The living cellular part of the hydrocaulus is a tubular coenosarc, composed of the three typical cnidarian layers surrounding the coelenteron (gastrovascular cavity). The protective covering of the hydrocaulus is a nonliving chitinous sheath, or perisarc. Attached to the hydrocaulus are individual polyp animals, or zooids. Most zooids are feeding polyps called hydranths, or gastrozooids. They may be tubular, bottle shaped, or vaselike, but all have a terminal mouth and a circle of tentacles. In some forms, such as Obelia, the perisarc continues as a protective cup around the polyp into which it can withdraw for protection (Figure 13-9). In others the polyp is naked (Figure 13-10). In some forms the perisarc is an inconspicuous, thin film.
Hydranths, much like miniature hydras, capture and ingest prey, such as tiny crustaceans, worms, and larvae, thus providing nutrition for the entire colony. After partial extracellular digestion in a hydranth, the digestive broth passes along the common gastrovascular cavity where it is taken up by gastrodermal cells, and intracellular digestion occurs.
Circulation within the gastrovascular cavity is a function of the ciliated gastrodermis but is also aided by rhythmical contractions and pulsations of the body, which occur in hydroids.
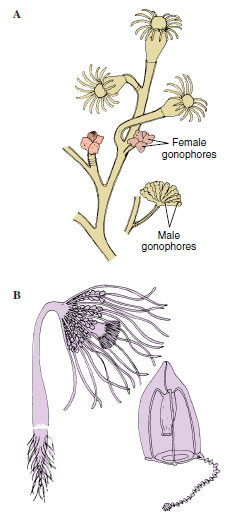 |
| Figure 13-10 Gymnoblastean hydroids. A, Eudendrium forms a bushy colony with naked hydranths and gonophores. There are no free medusae. B, Corymorpha is a solitary hydroid. It produces free-swimming medusae, each with a single trailing tentacle. |
Just as hydras reproduce asexually by budding, colonial hydroids bud off new individuals, thus increasing the size of the colony. New feeding polyps arise by budding, and medusa buds also arise on the colony. In Obelia these medusae bud from a reproductive polyp called a gonangium. The young medusae leave the colony as free-swimming individuals that mature and produce gametes (eggs and sperm) (Figure 13-9). In some species medusae remain attached to the colony and shed their gametes there. In other species medusae never develop and gametes are shed by male and female gonophores (Figure 13-10). Embryonation of the zygote results in a ciliated planula larva that swims about for a time. Then it settles down to a substratum to develop into a minute polyp that gives rise, by asexual budding, to the hydroid colony, thus completing the life cycle.
Hydroid medusae are usually smaller than scyphozoan medusae, ranging from 2 to 3 mm to several centimeters in diameter (Figure 13-11). The margin of the bell projects inward as a shelflike velum, which partly closes the open side of the bell and is used in swimming (Figure 13-12). Muscular pulsations that alternately fill and empty the bell propel the animal forward, aboral side first, with a weak “jet propulsion.” Tentacles attached to the bell margin are rich in nematocysts.
The mouth opening at the end of a suspended manubrium leads to a stomach and four radial canals that connect with a ring canal around the margin. This ring canal connects with the hollow tentacles. Thus the gastrovascular cavity is continuous from mouth to tentacles, and gastrodermis lines the entire system. Nutrition is similar to that of hydranths.
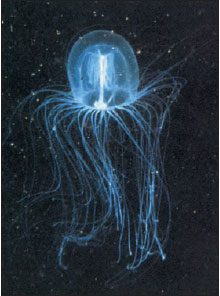 |
| Figure 13-11 Bell medusa, Polyorchis penicillatus, medusa stage of an unknown attached polyp. |
The nerve net is usually concentrated into two nerve rings at the base of the velum. The bell margin has a liberal supply of sensory cells. It usually also bears two kinds of specialized sense organs: statocysts, which are small organs of equilibrium (Figure 13-12B), and ocelli, which are light-sensitive organs.
Freshwater Medusae
The freshwater medusa Craspedacusta sowerbyi (Figure 13-13) (order Hydroida) may have evolved from marine ancestors in the Yangtze River of China. Probably introduced with shipments of aquatic plants, this interesting form has now been found in many parts of Europe, all over the United States, and in parts of Canada. Medusae may attain a diameter of 20 mm.
The polyp phase of this animal is tiny (2 mm) and has a very simple form with no perisarc and no tentacles. It occurs in colonies of a few polyps. For a long time its relation to the medusa was not recognized, and thus the polyp was given a name of its own, Microhydra ryderi. On the basis of its relationship to the jellyfish and the law of priority, both polyp and medusa should be called Craspedacusta (N.L. craspedon, velum,+ Gr. kystis, bladder).
The polyp has three methods of asexual reproduction, as shown in Figure 13-13.
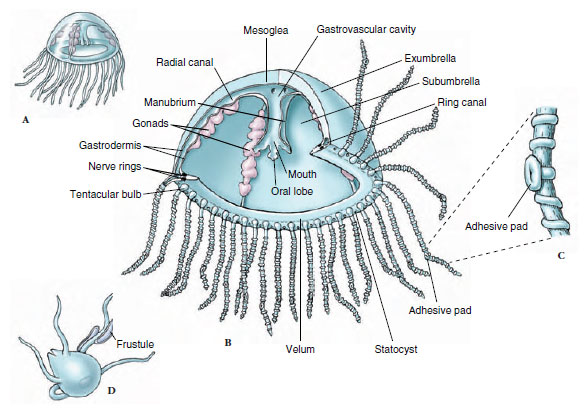 |
Figure 13-12 Structure of Gonionemus. A, Medusa with typical tetramerous arrangement. B, Cutaway view showing morphology. C, Portion of a tentacle with its adhesive pad and ridges of nematocysts. D, Tiny polyp, or hydroid stage, that develops from the planula larva. It can produce more polyps by budding (frustules) or produce medusa buds. |
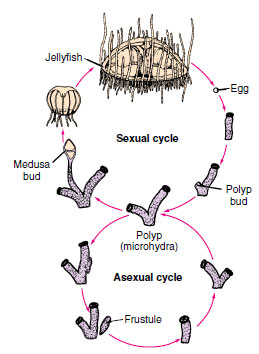 |
| Figure 13-13 Life cycle of Craspedacusta, a freshwater hydrozoan. The polyp has three methods of asexual reproduction: by budding off new individuals, which may remain attached to the parent (colony formation); by constricting off nonciliated planula-like larvae (frustules), which can move around and give rise to new polyps; and by producing medusa buds, which develop into sexual jellyfish. |
Other Hydrozoans
Members of orders Siphonophora and Chondrophora are among the most specialized of the Hydrozoa. They form polymorphic swimming or floating colonies containing several types of modified medusae and polyps.
Physalia (Gr. physallis, bladder), the Portuguese man-of-war (Figure 13-14), is one such colony with a rainbow-hued float of blues and pinks that carries it along the surface waters of tropical seas. Many are blown to shore on the eastern coast of the United States. The long, graceful tentacles, actually zooids, are laden with nematocysts and are capable of inflicting painful stings. The float, called a pneumatophore, is believed to have expanded from the original larval polyp. It contains a sac arising from the body wall and is filled with a gas similar to air. The float acts as a type of nurse-carrier for future generations of individuals that bud from it and hang suspended in the water. Some siphonophores, such as Stephalia and Nectalia, possess swimming bells as well as a float.
There are several types of polyp individuals. Gastrozooids are feeding polyps with a single long tentacle arising from the base of each. Some of these long, stinging tentacles become separated from the feeding polyp and are called dactylozooids, or fishing tentacles. These tentacles sting prey and lift it to the lips of feeding polyps. Among the modified medusoid individuals are the gonophores, which are little more than sacs containing either ovaries or testes.
Other hydrozoans secrete massive calcareous skeletons that resemble true corals (Figure 13-15). They are sometimes called hydrocorals.
 |
| Figure 13-14 A Portuguese man-of-war colony, Physalia physalis (order Siphonophora, class Hydrozoa). Colonies often drift onto southern ocean beaches, where a hazard to bathers. Each colony of medusa and polyp types is integrated to act as one individual. As many as 1000 zooids may be found in one colony. The nematocysts secrete a powerful neurotoxin. |
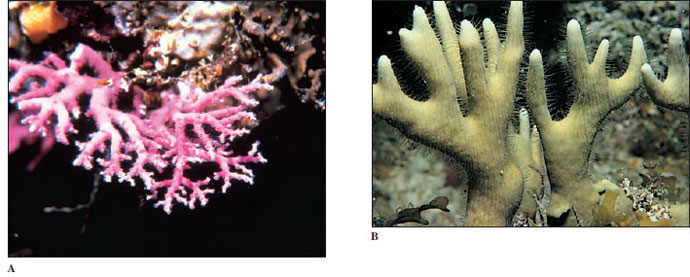 |
| Figure 13-15 These hydrozoans form calcareous skeletons that resemble true coral. A, Stylaster roseus (order Stylasterina) occurs commonly in caves and crevices in coral reefs. These fragile colonies branch in only a single plane and may be white, pink, purple, red, or red with white tips. B, Species of Millepora (order Milleporina) form branching or platelike colonies and often grow over the horny skeleton of gorgonians, as is shown here. They have a generous supply of powerful nematocysts that produce a burning sensation on human skin, justly earning the common name fire coral. |
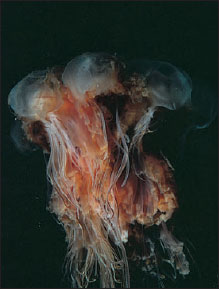 |
| Figure 13-16 Giant jellyfish, Cyanea capillata (order Semaeostomeae, class Scyphozoa). A North Atlantic species of Cyanea reaches a bell diameter exceeding 2 m. It is known as the “sea blubber” by fishermen. |
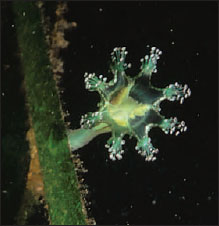 |
| Figure 13-17 Thaumatoscyphus hexaradiatus (order Stauromedusae, class Scyphozoa). Members of this order are unusual scyphozoans in that the medusae are sessile and attached to seaweed or other objects. |
Class Scyphozoa
Class Scyphozoa (si-fo-zo´a) (Gr. skyphos, cup) includes most of the larger jellyfishes, or “cup animals.” A few, such as Cyanea (Gr. kyanos, darkblue substance), may attain a bell diameter exceeding 2 m and tentacles 60 to 70 m long (Figure 13-16). Most scyphozoans, however, range from 2 to 40 cm in diameter. Most are found floating in the open sea, some even at depths of 3000 m, but one unusual order is sessile and attaches by a stalk to seaweeds and other objects on the sea bottom (Figure 13-17). Their coloring may range from colorless to striking orange and pink hues.
Bells of different species vary in depth from a shallow saucer shape to a deep helmet or goblet shape. The jelly (mesoglea) layer is unusually thick, giving the bell a fairly firm consistency. Despite the bell’s firmness the jelly is 95% to 96% water. Unlike hydromedusae, this layer in scyphomedusae also contains ameboid cells and fibers. Movement is by rhythmical pulsations of the umbrella. There is no velum as in hydromedusae. Tentacles may be numerous or few, and they may be short as in Aurelia (L. aurum, gold) or long as in Cyanea. Aurelia aurita (Figures 13-18 and 13-19) is a familiar species 7 to 10 cm in diameter, commonly found in the waters off both the east and west coasts of the United States, and widely used for study.
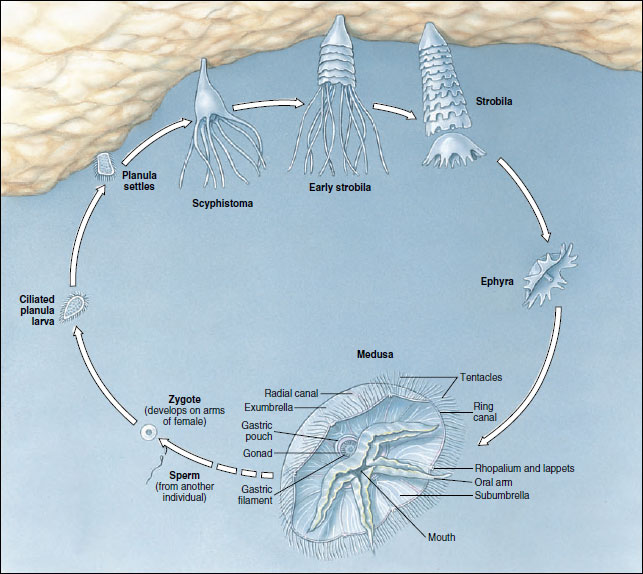 |
| Figure 13-18 Life cycle of Aurelia, a marine scyphozoan medusa. |
 |
| Figure 13-19 Moon jellyfish Aurelia aurita (class Scyphozoa) is comsmopolitan in distribution. It feeds on planktonic organisms caught in mucus on its umbrella |
The mouth is centered on the subumbrella side. The manubrium usually forms four frilly oral arms that are used in capturing and ingesting prey.
Tentacles, manubrium, and often the entire body surface are well supplied with nematocysts that can deliver painful stings. However, the primary function of scyphozoan nematocysts is not to attack humans but to paralyze prey animals, which are conveyed to the mouth lobes with the help of other tentacles or by the bending of the umbrella margin.
Aurelia, which has comparatively short tentacles, feeds on small planktonic animals. These are caught in mucus of the umbrella surface, are carried to “food pockets” on the umbrella margin by cilia, and are picked up from the pockets by the oral lobes whose cilia carry the food to the gastrovascular cavity. Cilia in the gastrodermis layer keep a current of water moving to bring food and oxygen into the stomach and expel wastes.
Cassiopeia (L. mythical queen of Ethiopia), the “upside-down jellyfish” common to Florida waters, and Rhizostoma (Gr. rhiza, root,+ stoma, mouth), which can be found in colder temperatures, belong to a group differing from that of Aurelia both in their lack of tentacles on the umbrella margin and in the structure of the oral arms. During development, edges of the oral lobes fold over and fuse, forming canals (arms or brachial canals) that become highly branched. These canals open to the surface at frequent intervals by pores called “mouths”; the original mouth is obliterated in the fusion of the oral lobes. Planktonic organisms caught in the mucus of the frilly oral arms are transported by cilia to the mouths and then up the brachial canals to the gastric cavity. In contrast to the usual swimming habit of medusae, Cassiopeia is usually found lying on its “back” in shallow lagoons. Its umbrella margin contracts about 20 times a minute, creating water currents to bring plankton into contact with the mucus and nematocysts of its oral lobes. Its tissues are abundantly supplied with symbiotic algal cells (zoo-xanthellae). As they lie sunning themselves in shallow water, Cassiopeia are thus reminiscent of large flowers in more ways than one.
Internally, extending out from the stomach of scyphozoans are four gastric pouches in which gastrodermis extends down in little tentacle-like projections called gastric filaments. These filaments are covered with nematocysts to quiet further any prey that may still be struggling. Gastric filaments are lacking in hydromedusae. A complex system of radial canals branches out from the pouches to a ring canal in the margin and forms a part of the gastrovascular cavity.
The nervous system in scyphozoans is a nerve net, with a subumbrella net that controls bell pulsations and another, more diffuse net that controls local reactions such as feeding.
Sexes are separate, with gonads located in the gastric pouches. Fertilization is internal, with sperm being carried by ciliary currents into the gastric pouch of the female. Zygotes may develop in seawater or may be brooded in folds of the oral arms. The ciliated planula larva becomes attached and develops into a scyphistoma, a hydralike form (Figure 13-18). By a process of strobilation the scyphistoma of Aurelia forms a series of saucerlike buds, ephyrae, and is now called a strobila (Figure 13-18). When the ephyrae break loose, they grow into mature jellyfish.
Class Cubozoa
The Cubozoa until recently were considered an order (Cubomedusae) of Scyphozoa. The medusoid is the predominant form (Figure 13-20); the polypoid is inconspicuous and in most cases unknown. Some cubozoan medusae may range up to 25 cm tall, but most are about 2 to 3 cm. In transverse section the bells are almost square. A tentacle or group of tentacles is found at each corner of the square at the umbrella margin. The base of each tentacle is differentiated into a flattened, tough blade called a pedalium (Figure 13-20). Rhopalia are present. The umbrella margin is not scalloped, and the subumbrella edge turns inward to form a velarium. The velarium functions as a velum does in hydrozoan medusae, increasing swimming efficiency, but it differs structurally. Cubomedusae are strong swimmers and voracious predators, feeding mostly on fish. Stings of some species can be fatal to humans.
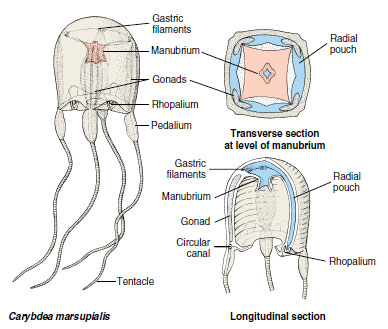 |
| Figure 13-20 Carybdea, a cubozoan medusa. |
The complete life cycle is known for only one species, Tripedalia cystophora (L. tri, three, + Gr. pedalion, rudder). The polyp is tiny (1 mm tall), solitary, and sessile. New polyps bud laterally, detach and creep away. Polyps do not produce ephyrae but metamorphose directly into medusae.
Class Anthozoa
Anthozoans, or “flower animals,” are polyps with a flowerlike appearance (Figure 13-21). There is no medusa stage. Anthozoa are all marine and are found in both deep and shallow water and in polar seas as well as tropical seas. They vary greatly in size and may be solitary or colonial. Many forms are supported by skeletons.
 |
| Figure 13-21 Sea anemones are the familiar and colorful “flower animals” of tide pools, rocks, and pilings of the intertidal zone. Most, however, are subtidal, their beauty seldom revealed to human eyes. These are rose anemones. Tealia piscivora (subclass Zoantharia, class Anthozoa). |
The class has three subclasses: Zoantharia (or Hexacorallia), containing sea anemones, hard corals, and others; Ceriantipatharia, containing only tube anemones and thorny corals; and Alcyonaria (or Octocorallia), containing soft and horny corals, such as sea fans, sea pens, sea pansies, and others. Zoantharians and ceriantipatharians have a hexamerous plan (of six or multiples of six) or polymerous symmetry and have simple tubular tentacles arranged in one or more circlets on the oral disc. Alcyonarians are octomerous (built on a plan of eight) and always have eight pinnate (featherlike) tentacles arranged around the margin of the oral disc (Figure 13-22).
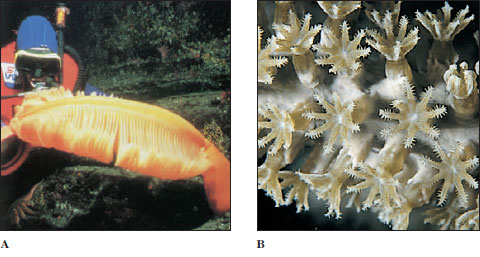 |
| Figure 13-22 A, Orange sea pen Ptilosarcus gurneyi (order Pennatulacea, class Anthozoa). Sea pens are colonial forms that inhabit soft bottoms. The base of the fleshy body of the primary polyp is buried in the bottom. It gives rise to numerous secondary, branching polyps. B, Close-up of a gorgonian. The pinnate tentacles characteristic of the subclass Alcyonaria are apparent. |
The gastrovascular cavity is large and partitioned by septa, or mesenteries, that are inward extensions of the body wall. Where one septum extends into the gastrovascular cavity from the body wall, another extends from the diametrically opposite side; thus, they are said to be coupled. In Zoantharia, the septa are not only coupled, they are also paired (Figure 13-23). The muscular arrangement varies among the different groups, but usually features circular muscles in the body wall and longitudinal and transverse muscles in the septa.
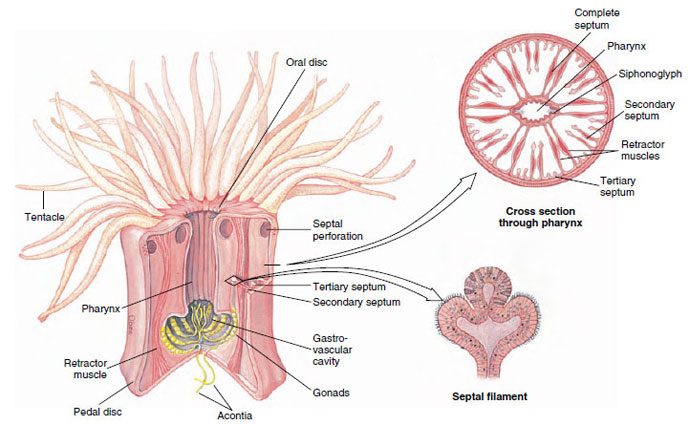 |
| Figure 13-23 Structure of a sea anemone. Free edges of septa and acontia threads are equipped with nematocysts to complete paralyzation of prey begun by the tentacles. |
The mesoglea is a mesenchyme containing ameboid cells. A general tendency towards biradial symmetry in the septal arrangement and occurs in the shape of the mouth and pharynx. There are no special organs for respiration or excretion.
Sea Anemones
Sea anemone (order Actiniaria) polyps are larger and heavier than hydrozoan polyps (Figure 13-21). Most range from 5 mm or less to 100 mm in diameter, and from 5 mm to 200 mm long, but some grow much larger. Some sea anemones are quite colorful. Anemones are found in coastal areas all over the world, especially in warmer waters. They attach by means of their pedal discs to shells, rocks, timber, or whatever submerged substrata they can find. Some burrow in mud or sand.
Sea anemones are cylindrical in form with a crown of tentacles arranged in one or more circles around the mouth of the flat oral disc (Figure 13-23). The slit-shaped mouth leads into a pharynx. At one or both ends of the mouth is a ciliated groove called a siphonoglyph, which extends into the pharynx. The siphonoglyph creates a water current directed into the pharynx. Cilia elsewhere on the pharynx direct water outward. Currents thus created carry in oxygen and remove wastes. They also help maintain an internal fluid pressure, providing a hydrostatic skeleton that serves in lieu of a true skeleton as a support for opposing muscles.
The pharynx leads into a large gastrovascular cavity that is divided into six radial chambers by means of six pairs of primary (complete) septa, or mesenteries, extending vertically from the body wall to the pharynx (Figure 13-23). Openings between chambers (septal perforations) in the upper part of the pharyngeal region help in water circulation. Smaller (incomplete) septa partially subdivide the large chambers and provide a means of increasing the surface area of the gastrovascular cavity. The free edge of each incomplete septum forms a type of sinuous cord called a septal filament that is provided with nematocysts and with gland cells for digestion. In some anemones (such as Metridium) the lower ends of the septal filaments are prolonged into acontia threads, also provided with nematocysts and gland cells, that can be protruded through the mouth or through pores in the body wall to help overcome prey or provide defense. The pores also aid in rapid discharge of water from the body when the animal is endangered and contracts to a small size.
Sea anemones are carnivorous, feeding on fish or almost any live (and sometimes dead) animals of suitable size. Some species live on minute forms caught by ciliary currents.
Feeding behavior in many zoantharians is under chemical control. Some respond to reduced glutathione. In certain others two compounds are involved: asparagine, the feeding activator, causes a bending of tentacles toward the mouth; then reduced glutathione induces swallowing of food.
Muscles are well developed in sea anemones, but the arrangement is quite different from that in Hydrozoa. Longitudinal fibers of the epidermis occur only in the tentacles and oral disc of most species. The strong longitudinal muscles of the column are gastrodermal and are located in the septa (Figure 13-23). Gastrodermal circular muscles in the column are well developed.
Most anemones can glide along slowly on their pedal discs. They can expand and stretch their tentacles in search of small vertebrates and invertebrates, which they overpower with tentacles and nematocysts and carry to the mouth. When disturbed, sea anemones contract and withdraw their tentacles and oral discs. Some anemones are able to swim to a limited extent by rhythmical bending movements, which may be a mechanism for escape from enemies such as sea stars and nudibranchs. Stomphia, for example, at the touch of a predatory sea star, will detach its pedal disc and make creeping or swimming movements to escape (Figure 13-24). This escape reaction is elicited not only by the touch of the star but also by exposure to drippings exuded by the star or to crude extracts made from its tissues. The sea drippings contain steroid saponins that are toxic and irritating to most invertebrates. Extracts from nudibranchs also can provoke this reaction in sea anemones.
 |
| Figure 13-24 A sea anemone that swims. When attacked by a predatory sea star Dermasterias, the anemone Stomphia didemon (subclass Zoantharia, class Anthozoa) detaches from the bottom and rolls or swims spasmodically to a safer location. |
Anemones form some interesting mutualistic relationships with other organisms. Many species harbor symbiotic algae (zooxanthellae) within their tissues, similar to the hard coralzooxanthellae association (described later in the section), and the anemones profit from the product of algal photosynthesis. Some anemones habitually attach to the shells occupied by certain hermit crabs. The hermit encourages the relationship and, finding its favorite species, which it recognizes by touch, it massages the anemone until it detaches. The hermit crab holds the anemone against its own shell until the anemone is firmly attached. The crab derives some protection against predators by the anemone. The anemone gets free transportation and particles of food dropped by the hermit crab.
Certain damselfishes (anemone fishes) (family Pomacentridae) form associations with large anemones, especially in tropical Indo-Pacific waters (Figure 13-25). An unknown property of the skin mucus of the fish causes the anemone’s nematocysts not to discharge, but if some other fish is so unfortunate as to brush the anemone’s tentacles, it is likely to become a meal. The anemone obviously provides shelter for the anemone fish, and the fish may help ventilate the anemone by its movements, keep the anemone free of sediment, and even lure an unwary victim to seek the same shelter.
 |
| Figure 13-25 Orangefin anemone fish (Amphiprion chrysopterus) nestles in the tentacles of its sea anemone host. Anemone fishes do not elicit stings from their hosts but may lure unsuspecting other fish to become meals for the anemone. |
Sexes are separate in some sea anemones, and some are hermaphroditic. Monoecious species are protandrous (produce sperm first, then eggs). Gonads are arranged on the margins of the septa, and fertilization takes place externally or in the gastrovascular cavity. The zygote develops into a ciliated larva. Asexual reproduction commonly occurs by pedal laceration or by longitudinal fission, occasionally by transverse fission or by budding. In pedal laceration, small pieces of the pedal disc break off as the animal moves, and each of these regenerates a small anemone.
Zoantharian Corals
The zoantharian corals belong to the order Scleractinia, sometimes known as the true or stony corals. The stony corals might be described as miniature sea anemones that live in calcareous cups they themselves have secreted (Figures 13-26 and 13-27). Like that of the anemones, a coral polyp’s gastrovascular cavity is subdivided by septa arranged in multiples of six (hexamerous) and its hollow tentacles surround the mouth, but there is no siphonoglyph.
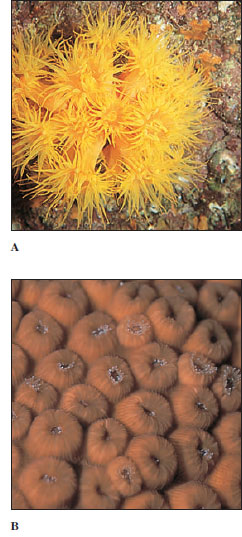  |
| Figure 13-26 A, Cup coral Tubastrea sp. The polyps form clumps resembling groups of sea anemones. Although often found on coral reefs, Tubastrea is not a reef-building coral (ahermatypic) and has no symbiotic zooxanthellae in its tissues. B, The polyps of Montastrea cavernosa are tightly withdrawn in the daytime but open to feed at night, as in C. (subclass Zoantharia) |
Instead of a pedal disc, the epidermis at the base of the column secretes a limy skeletal cup, including sclerosepta, which project up into the polyp between its true septa (Figure 13-27). Living polyps can retract into the safety of their cup when not feeding. Since the skeleton is secreted below the living tissue rather than within it, the calcareous material is an exoskeleton. In many colonial corals, the skeleton may become massive, building up over many years, with the living coral forming a sheet of tissue over the surface (Figure 13-28). The gastrovascular cavities of the polyps are all connected through this sheet of tissue. Three other small orders of Zoantharia are recognized.
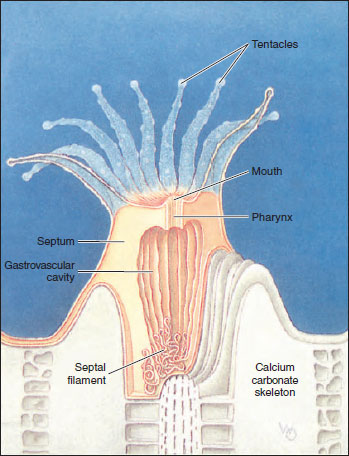 |
| Figure 13-27 Polyp of a zoantharian coral (order Scleractinia) showing calcareous cup (exoskeleton), gastrovascular cavity, sclerosepta, septa, and septal filaments. |
 |
| Figure 13-28 Boulder star coral, Montastrea annularis,(subclass Zoantharia, class Anthoza). Colonies can grow up to 10 feet (3 m) high. |
 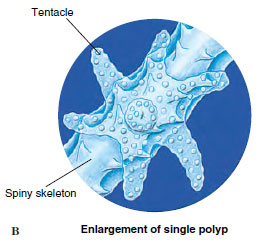 |
| Figure 13-30 A, Colony of Antipathes, a black or thorny coral (order Antipatharia, subclass Ceriantipatharia class Anthozoa). Most abundant in deep waters in the tropics, black corals secrete a tough, proteinaceous skeleton that can be worked into jewelry. B, The polyps of Antipatharia have six simple, nonretractile tentacles. The spiny processes in the skeleton are the origin of the common name thorny corals. |
 |
| Figure 13-29 A tube anemone (subclass Ceriantipatharia, order Ceriantharia) extends from its tube at night. Its oral disc bears long tentacles around the margin and short tentacles around the mouth. |
Tube Anemones and Thorny Corals
Members of subclass Ceriantipatharia have coupled but unpaired septa. Tube anemones (order Ceriantharia) (Figure 13-29) are solitary and live buried to the level of the oral disc in soft sediments. They occupy tubes constructed of se-creted mucus and threads of nematocyst-like organelles, into which they can withdraw. Thorny or black corals (order Antipatharia) (Figure 13-30) are colonial and attached to a firm substratum. Their skeleton is of a horny material and has thorns. Both of these orders are small in numbers of species and are limited to warmer waters of the sea.
Alcyonarian Corals
Alcyonarians (Octocorallia) have strict octomerous symmetry, with eight pinnate tentacles and eight unpaired, complete septa (Figure 13-22). They are all colonial, and gastrovascular cavities of the polyps communicate through a system of gastrodermal tubes called solenia (Figure 13-31). The solenia run through an extensive mesoglea (coenenchyme) in most alcyonarians, and the surface of the colony is covered by epidermis. The skeleton is secreted in the coenenchyme and consists of limy spicules, fused spicules, or a horny protein, often in combination. Thus the skeletal support of most alcyonarians is an endoskeleton. The variation in pattern among the species of alcyonarians lends great variety to the form of the colonies: from soft corals such as Dendronephthya (Figure 13-32), with their spicules scattered through the coenenchyme, to the tough, axial supports of sea fans and other gorgonian corals (Figure 13-33), to the fused spicules of organ-pipe coral. Renilla (L. ren, kidney, + illa, suffix), the sea pansy, is a colony reminiscent of a pansy flower. Its polyps are embedded in the fleshy upper side, and a short stalk that supports the colony is embedded in the sea floor. Ptilosarcus (Gr. ptilon, feather, + sarkos, flesh), a sea pen, is a member of the same order and may reach a length of 50 cm (Figure 13-22).
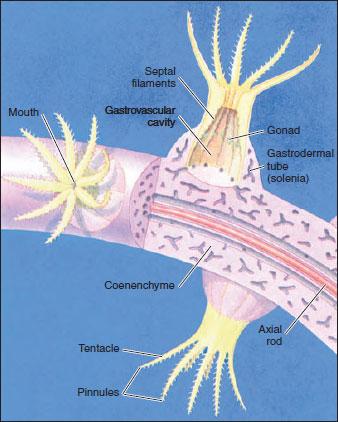 |
| Figure 13-31 Polyps of an alcyonarian coral (octocoral). Note the eight pinnate tentacles, coenenchyme, and solenia. They have an endoskeleton of limy spicules often with a horny protein, which may be in the form of an axial rod. |
The graceful beauty of alcyonarians— in hues of yellow, red, orange, and purple—helps create the “submarine gardens” of the coral reefs.
 |
| Figure 13-32 A soft coral, Dendronephthya sp. (order Alcyonacea, subclass Alcyonaria class Anthozoa), on a Pacific coral reef. The showy hues of this soft coral vary from pink and yellow to bright red and contribute much color to Indo- Pacific reefs. |
 |
| Figure 13-33 Colonial gorgonian, or horny, corals (order Gorgonacea, subclass Alcyonaria, class Anthozoa) are conspicuous components of reef faunas. These examples are from the western Pacific. A, Red gorgonian Melithaea sp. B, A sea fan, Subergorgia mollis. C, Red whip coral, Ellisella sp. |
Coral Reefs
Most students will have seen photographs or movies giving a glimpse of the vibrant color and life found on coral reefs, and some may have been fortunate enough to visit a reef. Coral reefs are among the most productive of all ecosystems, and they have a diversity of life forms rivaled only by tropical rain forests. They are large formations of calcium carbonate (limestone) in shallow tropical seas laid down by living organisms over thousands of years; living plants and animals are confined to the top layer of reefs where they add calcium carbonate to that deposited by their predecessors. The most important organisms that precipitate calcium carbonate from seawater to form reefs are the scleractinian, hermatypic (reef-building) corals (Figure 13-28) and coralline algae. Not only do coralline algae contribute to the total mass of calcium carbonate, but their precipitation of the substance helps to hold the reef together. Some alcyonarians and hydrozoa (especially Millepora [L. mille, thousand, + porus, pore] spp., “fire coral,” Figure 13-15B) contribute in some measure to the calcareous material, and an enormous variety of other organisms contributes small amounts. However, hermatypic (Gr. herma, support, mound, + typos, type) corals seem essential to formation of large reefs, since such reefs do not occur where these corals cannot live.
Hermatypic corals require warmth, light, and the salinity of undiluted seawater. These requirements limit coral reefs to shallow waters between 30 degrees north and 30 degrees south latitude and excludes them from areas with upwelling of cold water or areas near major river outflows with attendant low salinity and high turbidity. These corals require light because they have mutualistic algae (zooxanthellae) living in their tissues. The microscopic zooxanthellae are very important to the corals; their photosynthesis and fixation of carbon dioxide furnish food molecules for their hosts, they recycle phosphorus and nitrogenous waste compounds that otherwise would be lost, and they enhance the ability of the coral to deposit calcium carbonate.
Several types of reefs are commonly recognized. Fringing reefs are close to a landmass with either no lagoon or a narrow lagoon between reef and shore. A barrier reef (Figure 13-34) runs roughly parallel to shore and has a wider and deeper lagoon than does a fringing reef. Atolls are reefs that encircle a lagoon but not an island. These types of reefs typically slope rather steeply into deep water at their seaward edge. Patch or bank reefs occur some distance back from the steep, seaward slope in lagoons of barrier reefs or atolls. The so-called Great Barrier Reef, extending 2027 km long and up to 145 km from shore off the northeast coast of Australia, is actually a complex of reef types.
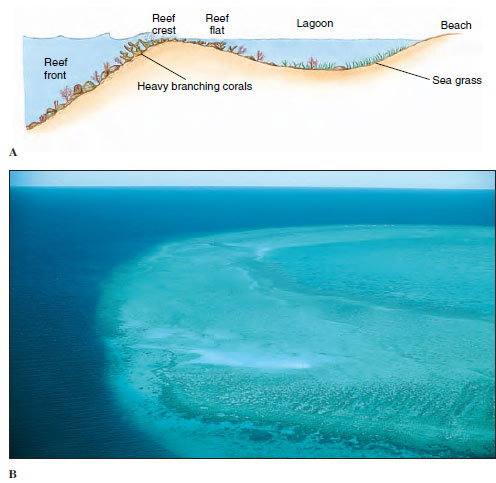 |
| Figure 13-34 A, Profile of a barrier reef. B, Portion of an atoll from the air. Reef slope plunges into deep water at left (dark blue), lagoon at right. |
Fringing, barrier, and atoll reefs all have distinguishable zones that are characterized by different groups of corals and other animals. The side of the reef facing the sea is the reef front or fore reef slope (Figure 13-34). The reef front is more or less parallel to the shore and perpendicular to the predominant direction of wave travel. It slopes downward into deeper water, sometimes gently at first, then precipitously. Characteristic assemblages of scleractinian corals grow deep on the slope, high near the crest, and in intermediate zones. In shallow water or slightly emergent at the top of the reef front is a reef crest. The upper front and the crest bear the greatest force of the waves and must absorb great energy during storms. Pieces of coral and other organisms are broken off at such times and thrown shoreward onto the reef flat, which slopes down into the lagoon. The reef flat thus receives a supply of calcareous material that is eventually broken down into coral sand. The sand is stabilized by the growth of plants such as turtle grass and coralline algae and ultimately becomes cemented into the mass of the reef by precipitation of carbonates. A reef is not an unbroken wall facing the sea but is highly irregular, with grooves, caves, crevices, channels through from the flat to the front, and deep, cup-shaped holes (“blue holes”). Alcyonarians tend to grow in these areas that are more protected from the full force of the waves, as well as on the flat and the deeper areas of the fore reef slope. Many other kinds of organisms inhabit cryptic locations such as caves and crevices.
Enormous numbers of species and individuals of invertebrate groups and fishes populate the reef ecosystem. For example, there are 300 common species of fishes on Caribbean reefs and more than 1200 on the Great Barrier Reef complex of Australia. It is marvelous that such diversity and productivity can be maintained, since reefs are washed by nutrient-poor waves of the open ocean. Although relatively little nutrient enters the ecosystem, little is lost because the interacting organisms are so efficient in recycling. The corals even feed on feces of fish swimming over them!




