Phylum Porifera: Sponges
Phylum Porifera: Sponges
Sponges belong to phylum Porifera (po-rif´-er-a) (L. porus, pore, + fera, bearing). Sponges bear myriads of tiny pores and canals that constitute a filterfeeding system adequate for their inactive life habit. They are sessile animals and depend on water currents carried through their unique canal systems to bring them food and oxygen and to carry away their body wastes. Their bodies are little more than masses of cells embedded in a gelatinous matrix and stiffened by a skeleton of minute spicules of calcium carbonate or silica and collagen. They have no organs or true tissues, and even their cells show a certain degree of independence. As sessile animals with only negligible body movement, they have not evolved a nervous system or sense organs and have only the simplest of contractile elements.
So, although they are multicellular, sponges share few of the characteristics of other metazoan phyla. They seem to be outside the line of evolution leading from choanoflagellates to other metazoa. For this reason they are often called Parazoa (Gr. para, beside or alongside of, + zoon, animal).
Sponges vary in size from a few millimeters to the great loggerhead sponges, which may reach 2 m or more across. Many sponge species are brightly colored because of pigments in their dermal cells. Red, yellow, orange, green, and purple sponges are not uncommon. However, color fades quickly when sponges are removed from water. Some sponges, including the simplest, are radially symmetrical, but many are quite irregular in shape. Some stand erect, some are branched or lobed, and others are low, even encrusting, in form (Figure 12-4). Some bore holes into shells or rocks.
Most of the 5000 or more sponge species are marine, although some 150 species live in fresh water. Marine sponges are abundant in all seas and at all depths, and a few even exist in brackish water. Although their embryos are free swimming, adults are always attached, usually to rocks, shells, corals, or other submerged objects. Some bottom-dwelling forms even grow on sand or mud. Their growth patterns often depend on shape of the substratum, direction and speed of water currents, and availability of space, so that the same species may differ markedly in appearance under different environmental conditions. Sponges in calm waters may grow taller and straighter than those in rapidly moving waters.
Many animals (crabs, nudibranchs, mites, bryozoans, and fish) live as commensals or parasites in or on sponges. Larger sponges particularly tend to harbor a large variety of invertebrate commensals. On the other hand, sponges grow on many other living animals, such as molluscs, barnacles, brachiopods, corals, or hydroids. Some crabs attach pieces of sponge to their carapace for camouflage and for protection, since most predators seem to find sponges distasteful. Some reef fishes, however, graze on shallow-water sponges.
Sponges are an ancient group, with an abundant fossil record extending back to the early Cambrian period and even, according to some claims, the Precambrian. Living poriferans traditionally have been assigned to three classes: Calcarea (with calcareous spicules), Hexactinellida (six-rayed siliceous spicules), and Demospongiae (with a skeleton of siliceous spicules or spongin [a specialized collagen] or both). A fourth class (Sclerospongiae) was erected to contain sponges with a massive calcareous skeleton and siliceous spicules. Some zoologists maintain that known species of sclerosponges can be placed in the traditional classes of sponges (Calcarea and Demospongiae); thus we do not need a new class.
Characteristics of Phylum Porifera
Form and Function
The only body openings of these unusual animals are pores, usually many tiny ones called ostia for incoming water, and a few large ones called oscula (sing., osculum) for water outlet. These openings are connected by a system of canals, some of which are lined with peculiar flagellated collar cells called choanocytes, whose flagella maintain a current of environmental water through the canals. Water enters the canals through a multitude of tiny incurrent pores (dermal ostia) and leaves by way of one or more large oscula. Choanocytes not only keep the water moving but also trap and phagocytize food particles that are carried in the water. Cells lining the passageways are very loosely organized. Collapse of the canals is prevented by the skeleton, which, depending on the species, may be composed of needlelike calcareous or siliceous spicules, a meshwork of organic spongin fibers, or a combination of the two.
Sessile animals make few movements and therefore need little in the way of nervous, sensory, or locomotor parts. Sponges apparently have been sessile from their earliest appearance and have never acquired specialized nervous or sensory structures, and they have only the very simplest of contractile systems.
Types of Canal Systems
Most sponges have one of three types of canal systems: asconoid, syconoid, or leuconoid (Figure 12-5).
Asconoids: Flagellated Spongocoels Asconoid sponges have the simplest organization. They are small and tube shaped. Water enters through microscopic dermal pores into a large cavity called a spongocoel, which is lined with choanocytes. Choanocyte flagella pull water through the pores and expel it through a single large osculum (see Figure 12-5). Leucosolenia (Gr. leukos, white, + solen, pipe) is an asconoid type of sponge. Its slender, tubular individuals grow in groups attached by a common stolon, or stem, to objects in shallow seawater. Clathrina (L. clathri, lattice work) is an asconoid with bright yellow, intertwined tubes (Figure 12-6). Asconoids are found only in the Calcarea.
Syconoids: Flagellated Canals Syconoid sponges look somewhat like larger editions of asconoids, from which they were derived. They have a tubular body and single osculum, but the body wall, which is thicker and more complex than that of asconoids, contains choanocyte-lined radial canals that empty into the spongocoel (see Figure 12-5). The spongocoel in syconoids is lined with epithelial-type cells rather than flagellated cells as in asconoids. Water enters through a large number of dermal ostia into incurrent canals and then filters through tiny openings called prosopyles into the radial canals (Figure 12-7). There food is ingested by the choanocytes, whose flagella force the water through internal pores (apo-pyles) into the spongocoel. From there it emerges through an osculum. Syconoids do not usually form highly branched colonies as asconoids do. During development, syconoid sponges pass through an asconoid stage; then flagellated canals form by evagination of the body wall. Their development provides evidence that syconoid sponges were derived from asconoid ancestral stock. Syconoids are found in classes Calcarea and Hexactinellida. Sycon (Gr. sykon, a fig) is a commonly studied example of the syconoid type of sponge (see Figure 12-5).
Leuconoids: Flagellated Chambers Leuconoid organization is the most complex of the sponge types and permits an increase in sponge size. Most leuconoids form large masses with numerous oscula (Figure 12-8). Clusters of flagellated chambers are filled from incurrent canals and discharge water into excurrent canals that eventually lead to the osculum (Figure 12-5). Most sponges are of the leuconoid type, which occurs in most Calcarea and in all other classes.
These three types of canal systems— asconoid, syconoid, and leuconoid— demonstrate an increase in complexity and efficiency of the water pumping system, but they do not imply an evolutionary or developmental sequence. The leuconoid grade of construction has evolved independently many times in sponges. Possession of a leuconoid plan is of clear adaptive value; it increases the proportion of flagellated surfaces compared with the volume, thus providing more collar cells to meet food demands.
Types of Cells
Sponge cells are loosely arranged in a gelatinous matrix called mesohyl (mesoglea, mesenchyme) (Figures 12-7 and 12-9). The mesohyl is the “connective tissue” of sponges; in it are found various ameboid cells, fibrils, and skeletal elements. Several types of cells occur in sponges.
Pinacocytes The nearest approach to a true tissue in sponges is arrangement of the pinacocyte cells of the pinacoderm (Figure 12-9). These are thin, flat, epithelial-type cells that cover the exterior surface and some interior surfaces. Some are T-shaped, with their cell bodies extending into the mesohyl. Pinacocytes are somewhat contractile and help regulate surface area of a sponge. Some pina-cocytes are modified as contractile myocytes, which are usually arranged in circular bands around oscula or pores, where they help regulate rate of water flow.
Choanocytes Choanocytes, which line flagellated canals and chambers, are ovoid cells with one end embedded in mesohyl and the other exposed. The exposed end bears a flagellum surrounded by a collar (Figures 12-9 and 12-10). Electron microscopy shows that the collar is made up of adjacent microvilli, connected to each other by delicate microfibrils, forming a fine filtering device for straining food particles from water (Figure 12-10B and C). The beat of a flagellum pulls water through the sievelike collar and forces it out through the open top of the collar. Particles too large to enter the collar become trapped in secreted mucus and slide down the collar to the base where they are phagocytized by the cell body. Larger particles have already been screened out by the small size of the dermal pores and prosopyles. Food engulfed by the cells is passed on to a neighboring archaeocyte for digestion.
Archaeocytes Archaeocytes are ameboid cells that move about in the mesohyl (Figure 12-9) and carry out a number of functions. They can phagocytize particles at the pinacoderm and receive particles for digestion from choanocytes. Archaeocytes apparently can differentiate into any of the other types of more specialized cells in the sponge. Some, called sclerocytes, secrete spicules. Others, called spongocytes, secrete the spongin fibers of the skeleton, and collencytes secrete fibrillar collagen. Lophocytes secrete large quantities of collagen but are distinguishable morphologically from collencytes.
Types of Skeletons
Its skeleton gives support to a sponge, preventing collapse of canals and chambers. The major structural protein in the animal kingdom is collagen, and fibrils of collagen are found throughout the intercellular matrix of all sponges. In addition, various Demospongiae secrete a form of collagen traditionally known as spongin. Several types of spongin, differing in chemical composition and form (fibers, spicules, filaments, spongin surrounding spicules, and so on) are found in various demosponges. Demospongiae also secrete siliceous spicules. Calcareous sponges secrete spicules composed mostly of crystalline calcium carbonate and have one, three, or four rays (Figure 12-11). Glass sponges have siliceous spicules with six rays arranged in three planes at right angles to each other. There are many variations in the shape of spicules, and these structural variations are of taxonomic importance.
Sponge Physiology
All activities of a sponge depend on the current of water flowing through its body. A sponge pumps a remarkable amount of water. Leuconia (Gr. leukos, white), for example, is a small leuconoid sponge about 10 cm tall and 1 cm in diameter. It is estimated that water enters through some 81,000 incurrent canals at a velocity of 0.1 cm/second. However, because Leuconia has more than 2 million flagellated chambers whose combined diameter is much greater than that of the canals, water flow through chambers slows to 0.001 cm/second. Such a flow rate allows ample opportunity for food capture by collar cells. All water is expelled through a single osculum at a velocity of 8.5 cm/second: a jet force capable of carrying waste products some distance away from the sponge. Some large sponges can filter 1500 liters of water a day.
Sponges feed primarily on particles suspended in the water pumped through their canal systems. Detritus particles, planktonic organisms, and bacteria are consumed nonselectively in the size range from 50 µm (average diameter of ostia) to 0.1 µm (width of spaces between microvilli of choanocyte collar). Pinacocytes may phagocytize particles at the surface, but most larger particles are consumed in the canals by archaeocytes that move close to the lining of the canals. The smallest particles, accounting for about 80% of the particulate organic carbon, are phagocytized by choanocytes. Sponges also absorb dissolved nutrients from the water passing through the system. Protein molecules are taken into choanocytes by pinocytosis.
Digestion is entirely intracellular (occurs within cells), and present evidence indicates that archaeocytes perform this chore. Choanocytes pass particles of food to archaeocytes for digestion.
There are no respiratory or excretory organs; both functions apparently occur by diffusion in individual cells. Contractile vacuoles are found in archaeocytes and choanocytes of freshwater sponges.
The only visible activities and responses in sponges, other than propulsion of water, are slight alterations in shape and closing and opening of incurrent and excurrent pores, and these movements are very slow. The most common response is closure of the oscula. Apparently excitation spreads from cell to cell, although some zoologists point to the possibility of coordination by means of substances carried in the water currents, and some zoologists have tried, not very successfully, to demonstrate presence of nerve cells.
Reproduction
Sponges reproduce both asexually and sexually. Asexual reproduction occurs by means of bud formation and by regeneration following fragmentation. External buds, after reaching a certain size, may become detached from the parent and float away to form new sponges, or they may remain to form colonies. Internal buds, or gemmules (Figure 12-12), are formed in freshwater sponges and some marine sponges. Here, archaeocytes collect in the mesohyl and become surrounded by a tough spongin coat incorporating siliceous spicules. When the parent animal dies, the gemmules survive and remain dormant, preserving the species during periods of freezing or severe drought. Later, cells in the gemmules escape through a special opening, the micropyle, and develop into new sponges. Gemmulation in freshwater sponges (Spongillidae) is thus an adaptation to changing seasons. Gemmules are also a means of colonizing new habitats, since they can spread by streams or animal carriers. What prevents gemmules from hatching during the season of formation rather than remaining dormant? Some species secrete a substance that inhibits early germination of gemmules, and gemmules do not germinate as long as they are held in the body of the parent. Other species undergo maturation at low temperatures (as in winter) before they germinate. Gemmules in marine sponges also seem to be an adaptation to pass the cold of winter; they are the only form in which Haliclona loosanoffi exists during the colder parts of the year in the northern part of its range.
In sexual reproduction most sponges are monoecious (have both male and female sex cells in one individual). Sperm arise from transformation of choanocytes. In Calcarea and at least some Demospongiae, oocytes also develop from choanocytes; in other demosponges oocytes apparently are derived from archaeocytes. Most sponges are viviparous; after fertilization the zygote is retained in and derives nourishment from the parent, and a ciliated larva is released. In such sponges, sperm are released into the water by one individual and taken into the canal system of another. There choanocytes phagocytize the sperm, then the choanocytes transform into carrier cells, which carry the sperm through the mesohyl to oocytes. Other sponges are oviparous, and both oocytes and sperm are expelled into the water. The free-swimming larva of most sponges is a solid-bodied parenchymula (Figure 12-13A). The outwardly directed, flagellated cells migrate to the interior after the larva settles and become choanocytes in the flagellated chambers.
Calcarea and a few Demospongiae have a very strange developmental pattern. A hollow blastula, called an amphiblastula (Figure 12-13B), develops, with flagellated cells toward the interior. The blastula then turns inside out (inversion), the flagellated ends of the cells becoming directed to the outside! Flagellated cells (micromeres) of the larva are at one end, and larger, nonflagellated cells (macromeres) are at the other. In contrast to other metazoan embryos, the micromeres invaginate into and are overgrown by the macromeres. The flagellated micromeres become choanocytes, archeocytes, and collencytes of the new sponge, and the nonflagellated cells give rise to pinacoderm and sclerocytes.
Regeneration and Somatic Embryogenesis
Sponges have a tremendous ability to repair injuries and to restore lost parts, a process called regeneration. Regeneration does not imply a reorganization of the entire animal, but only of the wounded portion.
On the other hand, if a sponge is cut into small fragments, or if the cells of a sponge are entirely dissociated and are allowed to fall into small groups, or aggregates, entire new sponges can develop from these fragments or aggregates of cells. This process has been termed somatic embryogenesis. Somatic embryogenesis involves a complete reorganization of the structure and functions of participating cells or bits of tissue. Isolated from influence of adjoining cells, they can realize their own potential to change in shape or function as they develop into a new organism.
A great deal of experimental work has been done in this field. The process of reorganization appears to differ in sponges of differing complexity. There is still some controversy concerning just what mechanisms cause adhesion of the cells and the share that each type of cell plays in the formative process.
Class Calcarea (Calcispongiae)
Calcarea (also called Calcispongiae) are calcareous sponges, so called because their spicules are composed of calcium carbonate. Spicules are straight (monaxons) or have three or four rays. These sponges tend to be small—10 cm or less in height—and tubular or vase shaped. They may be asconoid, syconoid, or leuconoid in structure. Though many are drab in color, some are bright yellow, red, green, or lavender. Leucosolenia and Sycon (often called Scypha or Grantia by biological supply companies) are marine shallowwater forms commonly studied in the laboratory. Leucosolenia is a small asconoid sponge that grows in branching colonies, usually arising from a network of horizontal, stolonlike tubes (Figure 12-6). Sycon is a solitary sponge that may live singly or form clusters by budding. The vase-shaped, typically syconoid animal is 1 to 3 cm long, with a fringe of straight spicules around the osculum to discourage small animals from entering.
Class Hexactinellida (Hyalospongiae): Glass Sponges
Glass sponges make up class Hexactinellida (or Hyalospongiae). Nearly all are deep-sea forms that are collected by dredging. Most are radially symmetrical, with vase- or funnelshaped bodies usually attached by stalks of root spicules to a substratum (Figure 12-11, Euplectella) (N. L. from Gr. euplektos, well plaited). They range from 7.5 cm to more than 1.3 m in length. Their distinguishing features are a skeleton of six-rayed siliceous spicules that are commonly bound together into a network forming a glasslike structure and a trabecular net of living tissue produced by the fusion of pseudopodia of archaeocytes. Within the trabecular net are elongated, finger-shaped chambers lined with choanocytes and opening into the spongocoel. The osculum is unusually large and may be covered by a sievelike plate of silica. There is no pinacoderm or gelatinous mesohyl, and both the external surface and the spongocoel are lined with a trabecular net. The skeleton is rigid, and muscular elements (myocytes) appear to be absent. The general arrangement of the chambers fits glass sponges into both syconoid and leuconoid types. Their structure is adapted to the slow, constant currents of sea bottoms, because channels and pores of the sponge wall are relatively large and uncomplicated and permit an easy flow of water. Little, however, is known about their physiology, doubtless because of their deep-water habitat.
The latticelike network of spicules found in many glass sponges is of exquisite beauty, such as that of Euplectella, or Venus’ flower basket (Figure 12-11), a classic example of Hexactinellida.
Class Demospongiae
Class Demospongiae contains 95% of living sponge species, including most larger sponges. Spicules are siliceous but are not six rayed, and they may be bound together by spongin or may be absent altogether. All members of the class are leuconoid, and all are marine except one family, the Spongillidae, or freshwater sponges.
Freshwater sponges are widely distributed in well-oxygenated ponds and streams, where they encrust plant stems and old pieces of submerged wood. They may resemble a bit of wrinkled scum, be pitted with pores, and be brownish or greenish in color. Common genera are Spongilla (L. spongia, from Gr. spongos, sponge) and Myenia. Freshwater sponges are most common in midsummer, although some are more easily found in the fall. They die and disintegrate in late autumn, leaving gemmules to produce the next year’s population. They also reproduce sexually.
Marine Demospongiae are quite varied and may be quite striking in color and shape (Figure 12-14). Some are encrusting; some are tall and fingerlike; some are low and spreading; some bore into shells; and some are shaped like fans, vases, cushions, or balls (Figure 12-14). Loggerhead sponges may grow several meters in diameter.
So-called bath sponges (Spongia, Hippospongia) belong to the group called horny sponges, which have spongin skeletons and lack siliceous spicules entirely.
Phylogeny and Adaptive Radiation
Phylogeny
Sponges originated before the Cambrian period. Two groups of calcareous spongelike organisms occupied early Paleozoic reefs. The Devonian period saw rapid development of many glass sponges. The possibility that sponges arose from choanoflagellates (protozoa that bear collars and flagella) earned support for a time. However, many zoologists opposed that hypothesis because sponges do not acquire collars until later in their embryological development. The outer cells of the larvae are flagellated but not collared, and they do not become collar cells until they become internal. Also, collar cells are found in certain corals and echinoderms, so they are not unique to the sponges.
However, these objections are countered by evidence based on the sequences of ribosomal RNA. This evidence supports the hypothesis that choanoflagellates and metazoans are sister groups. It suggests also that sponges and Eumetazoa are sister groups, with Porifera having separated before the origin of radiates and placozoans, but sharing a common ancestor.
Adaptive Radiation
Porifera are a highly successful group that includes several thousand species and a variety of marine and freshwater habitats. Their diversification centers largely on their unique water-current system and its various degrees of complexity. Proliferation of flagellated chambers in leuconoid sponges was more favorable to an increase in body size than that of asconoid and syconoid sponges because facilities for feeding and gaseous exchange were greatly enlarged.
Classification of Phylum Porifera
Class Calcarea (cal-ca´re-a) (L. calcis, lime) (Calcispongiae). Have spicules of calcium carbonate that often form a fringe around the osculum (main water outlet); spicules needle shaped or three or four rayed; all three types of canal systems (asconoid, syconoid, leuconoid) represented; all marine. Examples:
Class Hexactinellida (hex-ak-tin-el´i-da) (Gr. hex, six, + aktis, ray, + L. -ellus, dim. suffix) (Hyalospongiae). Have six-rayed, siliceous spicules extending at right angles from a central point; spicules often united to form network; body often cylindrical or funnel shaped; flagellated chambers in simple syconoid or leuconoid arrangement; habitat mostly deep water; all marine. Examples: Venus’ flower basket (Euplectella), Hyalonema.
Class Demospongiae (de-mo-spun´jee) (Gr. demos, people, + spongos, sponge). Have siliceous spicules that are not six rayed, or spongin, or both; leuconoid-type canal systems; one family found in fresh water; all others marine. Examples: Thenea, Cliona, Spongilla, Myenia, and all bath sponges.
Sponges belong to phylum Porifera (po-rif´-er-a) (L. porus, pore, + fera, bearing). Sponges bear myriads of tiny pores and canals that constitute a filterfeeding system adequate for their inactive life habit. They are sessile animals and depend on water currents carried through their unique canal systems to bring them food and oxygen and to carry away their body wastes. Their bodies are little more than masses of cells embedded in a gelatinous matrix and stiffened by a skeleton of minute spicules of calcium carbonate or silica and collagen. They have no organs or true tissues, and even their cells show a certain degree of independence. As sessile animals with only negligible body movement, they have not evolved a nervous system or sense organs and have only the simplest of contractile elements.
So, although they are multicellular, sponges share few of the characteristics of other metazoan phyla. They seem to be outside the line of evolution leading from choanoflagellates to other metazoa. For this reason they are often called Parazoa (Gr. para, beside or alongside of, + zoon, animal).
Sponges vary in size from a few millimeters to the great loggerhead sponges, which may reach 2 m or more across. Many sponge species are brightly colored because of pigments in their dermal cells. Red, yellow, orange, green, and purple sponges are not uncommon. However, color fades quickly when sponges are removed from water. Some sponges, including the simplest, are radially symmetrical, but many are quite irregular in shape. Some stand erect, some are branched or lobed, and others are low, even encrusting, in form (Figure 12-4). Some bore holes into shells or rocks.
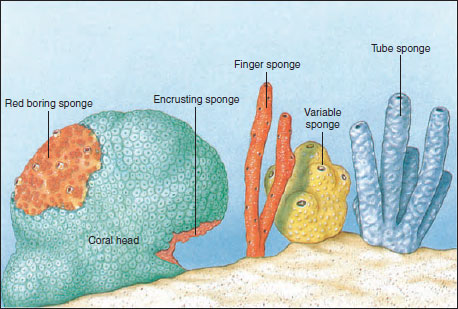 |
| Figure 12-4 Some growth habits and forms of sponges. |
Most of the 5000 or more sponge species are marine, although some 150 species live in fresh water. Marine sponges are abundant in all seas and at all depths, and a few even exist in brackish water. Although their embryos are free swimming, adults are always attached, usually to rocks, shells, corals, or other submerged objects. Some bottom-dwelling forms even grow on sand or mud. Their growth patterns often depend on shape of the substratum, direction and speed of water currents, and availability of space, so that the same species may differ markedly in appearance under different environmental conditions. Sponges in calm waters may grow taller and straighter than those in rapidly moving waters.
Many animals (crabs, nudibranchs, mites, bryozoans, and fish) live as commensals or parasites in or on sponges. Larger sponges particularly tend to harbor a large variety of invertebrate commensals. On the other hand, sponges grow on many other living animals, such as molluscs, barnacles, brachiopods, corals, or hydroids. Some crabs attach pieces of sponge to their carapace for camouflage and for protection, since most predators seem to find sponges distasteful. Some reef fishes, however, graze on shallow-water sponges.
Sponges are an ancient group, with an abundant fossil record extending back to the early Cambrian period and even, according to some claims, the Precambrian. Living poriferans traditionally have been assigned to three classes: Calcarea (with calcareous spicules), Hexactinellida (six-rayed siliceous spicules), and Demospongiae (with a skeleton of siliceous spicules or spongin [a specialized collagen] or both). A fourth class (Sclerospongiae) was erected to contain sponges with a massive calcareous skeleton and siliceous spicules. Some zoologists maintain that known species of sclerosponges can be placed in the traditional classes of sponges (Calcarea and Demospongiae); thus we do not need a new class.
Characteristics of Phylum Porifera
- Multicellular; body a loose aggregation of cells of mesenchymal origin>
- Body with pores (ostia), canals, and chambers that serve for passage of water
- Mostly marine; all aquatic
- Radial symmetry or none
- Epidermis of flat pinacocytes; most interior surfaces lined with flagellated collar cells (choanocytes) that create water currents; a gelatinous protein matrix called mesohyl (mesoglea) contains amebocytes of various types and skeletal elements
- Skeletal structure of fibrillar collagen (a protein) and calcareous or siliceous crystalline spicules, often combined with variously modified collagen (spongin)
- No organs or true tissues; digestion intracellular; excretion and respiration by diffusion
- Reactions to stimuli apparently local and independent; nervous system probably absent
- All adults sessile and attached to substratum
- Asexual reproduction by buds or gemmules and sexual reproduction by eggs and sperm; freeswimming ciliated larvae
Form and Function
The only body openings of these unusual animals are pores, usually many tiny ones called ostia for incoming water, and a few large ones called oscula (sing., osculum) for water outlet. These openings are connected by a system of canals, some of which are lined with peculiar flagellated collar cells called choanocytes, whose flagella maintain a current of environmental water through the canals. Water enters the canals through a multitude of tiny incurrent pores (dermal ostia) and leaves by way of one or more large oscula. Choanocytes not only keep the water moving but also trap and phagocytize food particles that are carried in the water. Cells lining the passageways are very loosely organized. Collapse of the canals is prevented by the skeleton, which, depending on the species, may be composed of needlelike calcareous or siliceous spicules, a meshwork of organic spongin fibers, or a combination of the two.
Sessile animals make few movements and therefore need little in the way of nervous, sensory, or locomotor parts. Sponges apparently have been sessile from their earliest appearance and have never acquired specialized nervous or sensory structures, and they have only the very simplest of contractile systems.
Types of Canal Systems
Most sponges have one of three types of canal systems: asconoid, syconoid, or leuconoid (Figure 12-5).
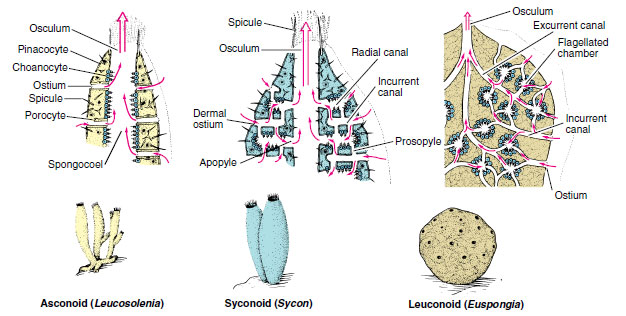 |
| Figure 12-5 Three types of sponge structure. The degree of complexity from simple asconoid to complex leuconoid type has involved mainly the water-canal and skeletal systems, accompanied by outfolding and branching of the collar-cell layer. The leuconoid type is considered the major plan for sponges, for it permits greater size and more efficient water circulation. |
Asconoids: Flagellated Spongocoels Asconoid sponges have the simplest organization. They are small and tube shaped. Water enters through microscopic dermal pores into a large cavity called a spongocoel, which is lined with choanocytes. Choanocyte flagella pull water through the pores and expel it through a single large osculum (see Figure 12-5). Leucosolenia (Gr. leukos, white, + solen, pipe) is an asconoid type of sponge. Its slender, tubular individuals grow in groups attached by a common stolon, or stem, to objects in shallow seawater. Clathrina (L. clathri, lattice work) is an asconoid with bright yellow, intertwined tubes (Figure 12-6). Asconoids are found only in the Calcarea.
 |
| Figure 12-6 Clathrina canariensis (class Calcarea) is common on Caribbean reefs in caves and under ledges. |
Syconoids: Flagellated Canals Syconoid sponges look somewhat like larger editions of asconoids, from which they were derived. They have a tubular body and single osculum, but the body wall, which is thicker and more complex than that of asconoids, contains choanocyte-lined radial canals that empty into the spongocoel (see Figure 12-5). The spongocoel in syconoids is lined with epithelial-type cells rather than flagellated cells as in asconoids. Water enters through a large number of dermal ostia into incurrent canals and then filters through tiny openings called prosopyles into the radial canals (Figure 12-7). There food is ingested by the choanocytes, whose flagella force the water through internal pores (apo-pyles) into the spongocoel. From there it emerges through an osculum. Syconoids do not usually form highly branched colonies as asconoids do. During development, syconoid sponges pass through an asconoid stage; then flagellated canals form by evagination of the body wall. Their development provides evidence that syconoid sponges were derived from asconoid ancestral stock. Syconoids are found in classes Calcarea and Hexactinellida. Sycon (Gr. sykon, a fig) is a commonly studied example of the syconoid type of sponge (see Figure 12-5).
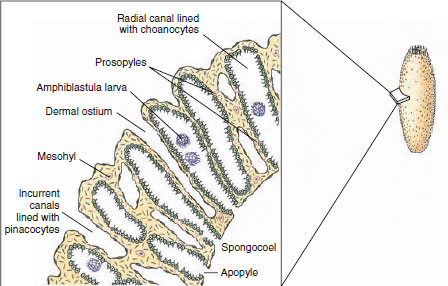 |
| Figure 12-7 Cross section through wall of sponge Sycon, showing canal system. |
Leuconoids: Flagellated Chambers Leuconoid organization is the most complex of the sponge types and permits an increase in sponge size. Most leuconoids form large masses with numerous oscula (Figure 12-8). Clusters of flagellated chambers are filled from incurrent canals and discharge water into excurrent canals that eventually lead to the osculum (Figure 12-5). Most sponges are of the leuconoid type, which occurs in most Calcarea and in all other classes.
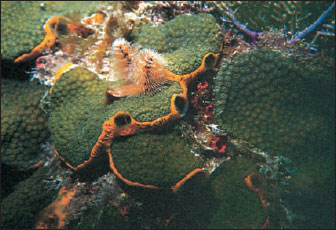 |
| Figure 12-8 This orange demosponge, Mycale laevis, often grows beneath platelike colonies of the stony coral, Montastrea annularis. The large oscula of the sponge are seen at the edges of the plates. Unlike some other sponges, Mycale does not burrow into the coral skeleton and may actually protect coral from invasion by more destructive species. Pinkish radioles of a Christmas tree worm, Spirobranchus giganteus (phylum Annelida, class Polychaeta) also project from the coral colony. An unidentified reddish sponge can be seen to the right of the Christmas tree worm. |
These three types of canal systems— asconoid, syconoid, and leuconoid— demonstrate an increase in complexity and efficiency of the water pumping system, but they do not imply an evolutionary or developmental sequence. The leuconoid grade of construction has evolved independently many times in sponges. Possession of a leuconoid plan is of clear adaptive value; it increases the proportion of flagellated surfaces compared with the volume, thus providing more collar cells to meet food demands.
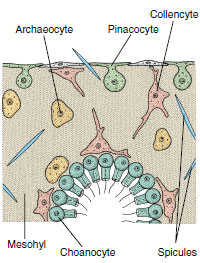 |
| Figure 12-9 Small section through sponge wall, showing four types of sponge cells. Pinacocytes are protective and contractile; choanocytes create water currents and engulf food particles; archaeocytes have a variety of functions; collencytes secrete collagen. |
Types of Cells
Sponge cells are loosely arranged in a gelatinous matrix called mesohyl (mesoglea, mesenchyme) (Figures 12-7 and 12-9). The mesohyl is the “connective tissue” of sponges; in it are found various ameboid cells, fibrils, and skeletal elements. Several types of cells occur in sponges.
Pinacocytes The nearest approach to a true tissue in sponges is arrangement of the pinacocyte cells of the pinacoderm (Figure 12-9). These are thin, flat, epithelial-type cells that cover the exterior surface and some interior surfaces. Some are T-shaped, with their cell bodies extending into the mesohyl. Pinacocytes are somewhat contractile and help regulate surface area of a sponge. Some pina-cocytes are modified as contractile myocytes, which are usually arranged in circular bands around oscula or pores, where they help regulate rate of water flow.
Choanocytes Choanocytes, which line flagellated canals and chambers, are ovoid cells with one end embedded in mesohyl and the other exposed. The exposed end bears a flagellum surrounded by a collar (Figures 12-9 and 12-10). Electron microscopy shows that the collar is made up of adjacent microvilli, connected to each other by delicate microfibrils, forming a fine filtering device for straining food particles from water (Figure 12-10B and C). The beat of a flagellum pulls water through the sievelike collar and forces it out through the open top of the collar. Particles too large to enter the collar become trapped in secreted mucus and slide down the collar to the base where they are phagocytized by the cell body. Larger particles have already been screened out by the small size of the dermal pores and prosopyles. Food engulfed by the cells is passed on to a neighboring archaeocyte for digestion.
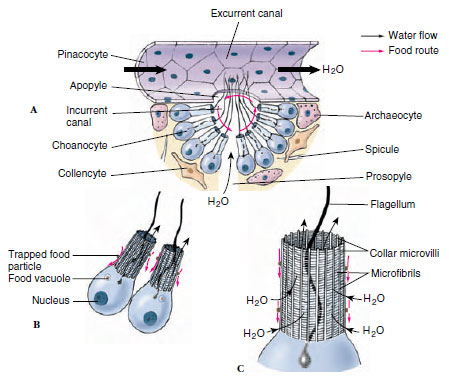 |
| Figure 12-10 Food trapping by sponge cells. A, Cutaway section of canals showing cellular structure and direction of water flow. B, Two choanocytes and C, structure of the collar. Small red arrows indicate movement of food particles. |
Archaeocytes Archaeocytes are ameboid cells that move about in the mesohyl (Figure 12-9) and carry out a number of functions. They can phagocytize particles at the pinacoderm and receive particles for digestion from choanocytes. Archaeocytes apparently can differentiate into any of the other types of more specialized cells in the sponge. Some, called sclerocytes, secrete spicules. Others, called spongocytes, secrete the spongin fibers of the skeleton, and collencytes secrete fibrillar collagen. Lophocytes secrete large quantities of collagen but are distinguishable morphologically from collencytes.
Types of Skeletons
Its skeleton gives support to a sponge, preventing collapse of canals and chambers. The major structural protein in the animal kingdom is collagen, and fibrils of collagen are found throughout the intercellular matrix of all sponges. In addition, various Demospongiae secrete a form of collagen traditionally known as spongin. Several types of spongin, differing in chemical composition and form (fibers, spicules, filaments, spongin surrounding spicules, and so on) are found in various demosponges. Demospongiae also secrete siliceous spicules. Calcareous sponges secrete spicules composed mostly of crystalline calcium carbonate and have one, three, or four rays (Figure 12-11). Glass sponges have siliceous spicules with six rays arranged in three planes at right angles to each other. There are many variations in the shape of spicules, and these structural variations are of taxonomic importance.
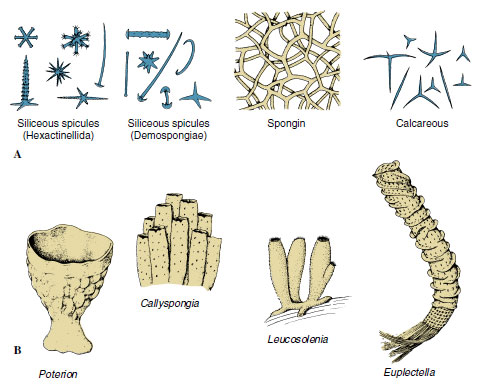 |
| Figure 12-11 A, Types of spicules found in sponges. An amazing diversity, complexity, and beauty of form occurs among the many types of spicules. B, Some sponge body forms. |
Sponge Physiology
All activities of a sponge depend on the current of water flowing through its body. A sponge pumps a remarkable amount of water. Leuconia (Gr. leukos, white), for example, is a small leuconoid sponge about 10 cm tall and 1 cm in diameter. It is estimated that water enters through some 81,000 incurrent canals at a velocity of 0.1 cm/second. However, because Leuconia has more than 2 million flagellated chambers whose combined diameter is much greater than that of the canals, water flow through chambers slows to 0.001 cm/second. Such a flow rate allows ample opportunity for food capture by collar cells. All water is expelled through a single osculum at a velocity of 8.5 cm/second: a jet force capable of carrying waste products some distance away from the sponge. Some large sponges can filter 1500 liters of water a day.
Sponges feed primarily on particles suspended in the water pumped through their canal systems. Detritus particles, planktonic organisms, and bacteria are consumed nonselectively in the size range from 50 µm (average diameter of ostia) to 0.1 µm (width of spaces between microvilli of choanocyte collar). Pinacocytes may phagocytize particles at the surface, but most larger particles are consumed in the canals by archaeocytes that move close to the lining of the canals. The smallest particles, accounting for about 80% of the particulate organic carbon, are phagocytized by choanocytes. Sponges also absorb dissolved nutrients from the water passing through the system. Protein molecules are taken into choanocytes by pinocytosis.
Digestion is entirely intracellular (occurs within cells), and present evidence indicates that archaeocytes perform this chore. Choanocytes pass particles of food to archaeocytes for digestion.
There are no respiratory or excretory organs; both functions apparently occur by diffusion in individual cells. Contractile vacuoles are found in archaeocytes and choanocytes of freshwater sponges.
The only visible activities and responses in sponges, other than propulsion of water, are slight alterations in shape and closing and opening of incurrent and excurrent pores, and these movements are very slow. The most common response is closure of the oscula. Apparently excitation spreads from cell to cell, although some zoologists point to the possibility of coordination by means of substances carried in the water currents, and some zoologists have tried, not very successfully, to demonstrate presence of nerve cells.
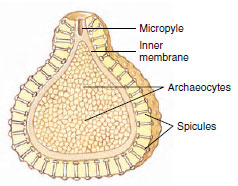 |
| Figure 12-12 Section through a gemmule of a freshwater sponge (Spongillidae). Gemmules are a mechanism for survival of the harsh conditions of winter. On return of favorable conditions, the archaeocytes exit through the micropyle to form a new sponge. The archaeocytes of the gemmule give rise to all the cell types of the new sponge structure. |
Reproduction
Sponges reproduce both asexually and sexually. Asexual reproduction occurs by means of bud formation and by regeneration following fragmentation. External buds, after reaching a certain size, may become detached from the parent and float away to form new sponges, or they may remain to form colonies. Internal buds, or gemmules (Figure 12-12), are formed in freshwater sponges and some marine sponges. Here, archaeocytes collect in the mesohyl and become surrounded by a tough spongin coat incorporating siliceous spicules. When the parent animal dies, the gemmules survive and remain dormant, preserving the species during periods of freezing or severe drought. Later, cells in the gemmules escape through a special opening, the micropyle, and develop into new sponges. Gemmulation in freshwater sponges (Spongillidae) is thus an adaptation to changing seasons. Gemmules are also a means of colonizing new habitats, since they can spread by streams or animal carriers. What prevents gemmules from hatching during the season of formation rather than remaining dormant? Some species secrete a substance that inhibits early germination of gemmules, and gemmules do not germinate as long as they are held in the body of the parent. Other species undergo maturation at low temperatures (as in winter) before they germinate. Gemmules in marine sponges also seem to be an adaptation to pass the cold of winter; they are the only form in which Haliclona loosanoffi exists during the colder parts of the year in the northern part of its range.
In sexual reproduction most sponges are monoecious (have both male and female sex cells in one individual). Sperm arise from transformation of choanocytes. In Calcarea and at least some Demospongiae, oocytes also develop from choanocytes; in other demosponges oocytes apparently are derived from archaeocytes. Most sponges are viviparous; after fertilization the zygote is retained in and derives nourishment from the parent, and a ciliated larva is released. In such sponges, sperm are released into the water by one individual and taken into the canal system of another. There choanocytes phagocytize the sperm, then the choanocytes transform into carrier cells, which carry the sperm through the mesohyl to oocytes. Other sponges are oviparous, and both oocytes and sperm are expelled into the water. The free-swimming larva of most sponges is a solid-bodied parenchymula (Figure 12-13A). The outwardly directed, flagellated cells migrate to the interior after the larva settles and become choanocytes in the flagellated chambers.
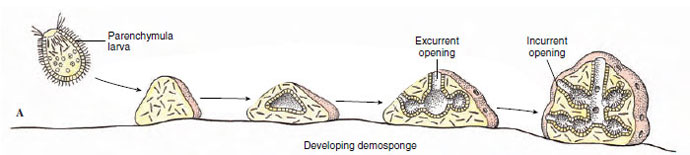 |
| Figure 12-13 A, Development of demosponges. B, Development of the syconoid sponge Sycon. |
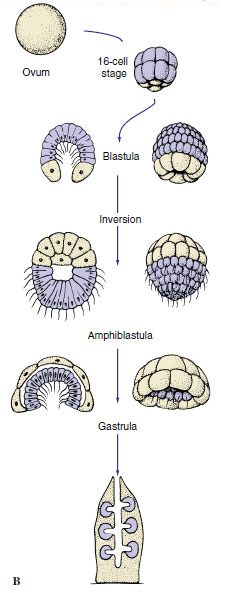 |
Calcarea and a few Demospongiae have a very strange developmental pattern. A hollow blastula, called an amphiblastula (Figure 12-13B), develops, with flagellated cells toward the interior. The blastula then turns inside out (inversion), the flagellated ends of the cells becoming directed to the outside! Flagellated cells (micromeres) of the larva are at one end, and larger, nonflagellated cells (macromeres) are at the other. In contrast to other metazoan embryos, the micromeres invaginate into and are overgrown by the macromeres. The flagellated micromeres become choanocytes, archeocytes, and collencytes of the new sponge, and the nonflagellated cells give rise to pinacoderm and sclerocytes.
Regeneration and Somatic Embryogenesis
Sponges have a tremendous ability to repair injuries and to restore lost parts, a process called regeneration. Regeneration does not imply a reorganization of the entire animal, but only of the wounded portion.
On the other hand, if a sponge is cut into small fragments, or if the cells of a sponge are entirely dissociated and are allowed to fall into small groups, or aggregates, entire new sponges can develop from these fragments or aggregates of cells. This process has been termed somatic embryogenesis. Somatic embryogenesis involves a complete reorganization of the structure and functions of participating cells or bits of tissue. Isolated from influence of adjoining cells, they can realize their own potential to change in shape or function as they develop into a new organism.
A great deal of experimental work has been done in this field. The process of reorganization appears to differ in sponges of differing complexity. There is still some controversy concerning just what mechanisms cause adhesion of the cells and the share that each type of cell plays in the formative process.
Class Calcarea (Calcispongiae)
Calcarea (also called Calcispongiae) are calcareous sponges, so called because their spicules are composed of calcium carbonate. Spicules are straight (monaxons) or have three or four rays. These sponges tend to be small—10 cm or less in height—and tubular or vase shaped. They may be asconoid, syconoid, or leuconoid in structure. Though many are drab in color, some are bright yellow, red, green, or lavender. Leucosolenia and Sycon (often called Scypha or Grantia by biological supply companies) are marine shallowwater forms commonly studied in the laboratory. Leucosolenia is a small asconoid sponge that grows in branching colonies, usually arising from a network of horizontal, stolonlike tubes (Figure 12-6). Sycon is a solitary sponge that may live singly or form clusters by budding. The vase-shaped, typically syconoid animal is 1 to 3 cm long, with a fringe of straight spicules around the osculum to discourage small animals from entering.
Class Hexactinellida (Hyalospongiae): Glass Sponges
Glass sponges make up class Hexactinellida (or Hyalospongiae). Nearly all are deep-sea forms that are collected by dredging. Most are radially symmetrical, with vase- or funnelshaped bodies usually attached by stalks of root spicules to a substratum (Figure 12-11, Euplectella) (N. L. from Gr. euplektos, well plaited). They range from 7.5 cm to more than 1.3 m in length. Their distinguishing features are a skeleton of six-rayed siliceous spicules that are commonly bound together into a network forming a glasslike structure and a trabecular net of living tissue produced by the fusion of pseudopodia of archaeocytes. Within the trabecular net are elongated, finger-shaped chambers lined with choanocytes and opening into the spongocoel. The osculum is unusually large and may be covered by a sievelike plate of silica. There is no pinacoderm or gelatinous mesohyl, and both the external surface and the spongocoel are lined with a trabecular net. The skeleton is rigid, and muscular elements (myocytes) appear to be absent. The general arrangement of the chambers fits glass sponges into both syconoid and leuconoid types. Their structure is adapted to the slow, constant currents of sea bottoms, because channels and pores of the sponge wall are relatively large and uncomplicated and permit an easy flow of water. Little, however, is known about their physiology, doubtless because of their deep-water habitat.
The latticelike network of spicules found in many glass sponges is of exquisite beauty, such as that of Euplectella, or Venus’ flower basket (Figure 12-11), a classic example of Hexactinellida.
Class Demospongiae
Class Demospongiae contains 95% of living sponge species, including most larger sponges. Spicules are siliceous but are not six rayed, and they may be bound together by spongin or may be absent altogether. All members of the class are leuconoid, and all are marine except one family, the Spongillidae, or freshwater sponges.
Freshwater sponges are widely distributed in well-oxygenated ponds and streams, where they encrust plant stems and old pieces of submerged wood. They may resemble a bit of wrinkled scum, be pitted with pores, and be brownish or greenish in color. Common genera are Spongilla (L. spongia, from Gr. spongos, sponge) and Myenia. Freshwater sponges are most common in midsummer, although some are more easily found in the fall. They die and disintegrate in late autumn, leaving gemmules to produce the next year’s population. They also reproduce sexually.
Marine Demospongiae are quite varied and may be quite striking in color and shape (Figure 12-14). Some are encrusting; some are tall and fingerlike; some are low and spreading; some bore into shells; and some are shaped like fans, vases, cushions, or balls (Figure 12-14). Loggerhead sponges may grow several meters in diameter.
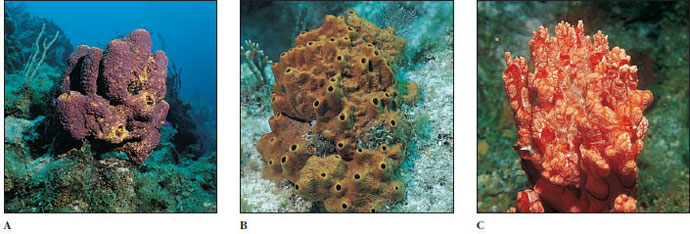 |
| Figure 12-14 Marine Demospongiae on Caribbean coral reefs. A, Pseudoceratina crassa is a colorful sponge growing at moderate depths. B, Ectyoplasia ferox is irregular in shape and its oscula form small, volcano-like cones. It is toxic and may cause skin irritation if touched. C, Monanchora unguifera with commensal brittle star, Ophiothrix suensoni (phylum Echinodermata, class Ophiuroidea). |
So-called bath sponges (Spongia, Hippospongia) belong to the group called horny sponges, which have spongin skeletons and lack siliceous spicules entirely.
Phylogeny and Adaptive Radiation
Phylogeny
Sponges originated before the Cambrian period. Two groups of calcareous spongelike organisms occupied early Paleozoic reefs. The Devonian period saw rapid development of many glass sponges. The possibility that sponges arose from choanoflagellates (protozoa that bear collars and flagella) earned support for a time. However, many zoologists opposed that hypothesis because sponges do not acquire collars until later in their embryological development. The outer cells of the larvae are flagellated but not collared, and they do not become collar cells until they become internal. Also, collar cells are found in certain corals and echinoderms, so they are not unique to the sponges.
However, these objections are countered by evidence based on the sequences of ribosomal RNA. This evidence supports the hypothesis that choanoflagellates and metazoans are sister groups. It suggests also that sponges and Eumetazoa are sister groups, with Porifera having separated before the origin of radiates and placozoans, but sharing a common ancestor.
Adaptive Radiation
Porifera are a highly successful group that includes several thousand species and a variety of marine and freshwater habitats. Their diversification centers largely on their unique water-current system and its various degrees of complexity. Proliferation of flagellated chambers in leuconoid sponges was more favorable to an increase in body size than that of asconoid and syconoid sponges because facilities for feeding and gaseous exchange were greatly enlarged.
Classification of Phylum Porifera
Class Calcarea (cal-ca´re-a) (L. calcis, lime) (Calcispongiae). Have spicules of calcium carbonate that often form a fringe around the osculum (main water outlet); spicules needle shaped or three or four rayed; all three types of canal systems (asconoid, syconoid, leuconoid) represented; all marine. Examples:
Class Hexactinellida (hex-ak-tin-el´i-da) (Gr. hex, six, + aktis, ray, + L. -ellus, dim. suffix) (Hyalospongiae). Have six-rayed, siliceous spicules extending at right angles from a central point; spicules often united to form network; body often cylindrical or funnel shaped; flagellated chambers in simple syconoid or leuconoid arrangement; habitat mostly deep water; all marine. Examples: Venus’ flower basket (Euplectella), Hyalonema.
Class Demospongiae (de-mo-spun´jee) (Gr. demos, people, + spongos, sponge). Have siliceous spicules that are not six rayed, or spongin, or both; leuconoid-type canal systems; one family found in fresh water; all others marine. Examples: Thenea, Cliona, Spongilla, Myenia, and all bath sponges.




