Structural and Functional Adaptations of Mammals
Structural and
Functional
Adaptations of
Mammals
Integument and Its Derivatives
Mammalian skin and especially its modifications distinguish mammals as a group. As the interface between an animal and its environment, the skin is strongly molded by the animal’s way of life. In general the skin is thicker in mammals than in other classes of vertebrates, although as in all vertebrates it is made up of epidermis and dermis (see Figure 30-5). Among mammals the dermis becomes much thicker than the epidermis. The epidermis is thinner where it is well protected by hair, but in places that are subject to much contact and use, such as the palms or soles, its outer layers become thick and cornified with keratin.
Hair
Hair is especially characteristic of mammals, although humans are not very hairy creatures and, in whales, hair is reduced to only a few sensory bristles on the snout. A hair grows from a hair follicle that, although an epidermal structure, is sunk into the dermis of the skin (Figure 30-5). The hair grows continuously by rapid proliferation of cells in the follicle. As the hair shaft is pushed upward, new cells are carried away from their source of nourishment and die, turning into the same dense type of fibrous protein, called keratin, that constitutes nails, claws, hooves, and feathers.
Mammals characteristically have two kinds of hair forming the pelage (fur coat): (1) dense and soft under hair for insulation and (2) coarse and longer guard hair for protection against wear and to provide coloration. Underhair traps a layer of insulating air. In aquatic mammals, such as fur seals, otters, and beavers, it is so dense that it is almost impossible to wet. In water, guard hairs become wet and mat down, forming a protective blanket over the underhair (Figure 30-6).
When a hair reaches a certain
length, it stops growing. Normally it
remains in the follicle until a new
growth starts, whereupon it falls out.
In most mammals there are periodic
molts of the entire coat. In humans,
hair is shed and replaced throughout
life (although balding males confirm
that replacement is not assured!).
In the simplest cases, such as foxes and seals, the coat is shed once every summer. Most mammals have two annual molts, one in the spring and one in the fall. Summer coats are always much thinner than winter coats and in some mammals it may be a different color. Several northern mustelid carnivores, for example, weasels, have white winter coats and brown-colored summer coats. It was once believed that the white inner pelage of arctic animals conserves body heat by reducing radiation loss; in fact, dark and white pelages radiate heat equally well. Winter white pelage of arctic animals is simply camouflage in a land of snow. The varying hare of North America has three annual molts: the white winter coat is replaced by a brownish gray summer coat, and this is replaced in autumn by a grayer coat, which is soon shed to reveal the winter white coat beneath (Figure 30-7). White fur of arctic mammals in winter (leukemism) is not to be confused with albinism, caused by a recessive gene that blocks pigment formation. Albinos have red eyes and pinkish skin, whereas arctic animals in their winter coats have dark eyes and often dark-colored ear tips, noses, and tail tips.
Outside the Arctic, most mammals wear somber colors that are protective. Often the species is marked with “saltand- pepper” coloration or a disruptive pattern that helps make it inconspicuous in its natural surroundings. Examples are the spots of leopards and fawns and the stripes of tigers. Skunks advertise their presence with conspicuous warning coloration.
The hair of mammals has become modified to serve many purposes. Bristles of hogs, spines of porcupines and their kin, and vibrissae on the snouts of most mammals are examples. Vibrissae, commonly called “whiskers,” are really sensory hairs that provide a tactile sense to many mammals. The slightest movement of a vibrissa generates impulses in sensory nerve endings that travel to special sensory areas in the brain. Vibrissae are especially long in nocturnal and burrowing animals.
Porcupines, hedgehogs, echidnas, and a few other mammals have developed an effective and dangerous spiny armor. When cornered, the common North American porcupine turns its back toward the attacker and lashes out with the barbed tail. The lightlyattached quills break off at their bases when they enter the skin and, aided by backward-pointed hooks on the tips, work deeply into tissues. Dogs are frequent victims (Figure 30-8) but fishers, wolverines, and bobcats are able to flip the porcupine onto its back to expose vulnerable underparts.
Horns and Antlers
Three kinds of horns or hornlike structures are found in mammals. True horns, found in ruminants (for example, sheep and cattle), are hollow sheaths of keratinized epidermis that embrace a core of bone arising from the skull. True horns are not normally shed, usually are not branched (although they may be greatly curved), grow continuously, and are found in both sexes. Horns may be absent from pronghorn antelope females but, if present, are shorter than those of the male.
Antlers of the deer family are branched and composed of solid bone when mature. During their annual spring growth, antlers develop beneath a covering of highly vascular soft skin called velvet (Figure 30-9). Except for caribou (Figure 30-16A), only males of the species produce antlers. When growth of the antlers is complete just before the fall breeding season, the blood vessels constrict and the stag removes the velvet by rubbing the antlers against trees. Antlers are shed after the breeding season. New buds appear a few months later to herald the next set of antlers. For several years each new pair of antlers is larger and more elaborate than the previous set. Annual growth of antlers places a strain on the mineral metabolism, since during the growing season an older moose or elk must accumulate 50 or more pounds of calcium salts from its vegetable diet.
The rhinoceros horn is the third kind of hornlike structure. Hairlike keratinized filaments that arise from dermal papillae are cemented together to form these structures, which are not attached to the skull.
Glands
Of all vertebrates, mammals have the greatest variety of integumentary glands. Most fall into one of four classes: sweat, scent, sebaceous, and mammary. All are derivatives of the epidermis (Figure 30-5).
Sweat glands are tubular, highly coiled glands that occur over much of the body surface in most mammals (Figure 30-5). They are not present in other vertebrates. There are two kinds of sweat glands: eccrine and apocrine. Eccrine glands secrete a watery fluid that, if evaporated on the skin’s surface, draws heat away from the skin and cools it. Eccrine glands occur in hairless regions, especially the foot pads, in most mammals, although in horses and most primates they are scattered over the body. They are either reduced or absent in rodents, rabbits, and whales. Apocrine glands are larger than eccrine glands and have longer and more convoluted ducts. Their secretory coil is in the dermis and extends deep into the hypodermis. They always open into a hair follicle or where a hair once was. Apocrine gland development occurs near puberty and is restricted (in the human species) to the axillae (armpits), mons pubis, breasts, prepuce, scrotum, and external auditory canals. In contrast to the watery secretions of eccrine glands, apocrine secretions are milky fluids, whitish or yellow in color, that dry on the skin to form a film. Apocrine glands are not involved in heat regulation. Their activity is correlated with certain aspects of the reproductive cycle.
Scent glands are present in nearly all mammals. Their location and functions vary greatly. They are used for communication with members of the same species, for marking territorial boundaries, for warning, or for defense. Scent-producing glands are located in orbital, metatarsal, and inter-digital regions (deer); behind the eyes and on the cheek (pica and woodchuck); penis (muskrats, beavers, and many canines); base of the tail (wolves and foxes); back of the head (dromedary); and anal region (skunks, minks, and weasels). The latter, the most odoriferous of all glands, open by ducts into the anus; their secretions can be discharged forcefully for 2 to 3 meters. During the mating season many mammals give off strong scents for attracting the opposite sex. Humans also are endowed with scent glands. However civilization has taught us to dislike our own scent, a concern that has stimulated a lucrative deodorant industry to produce an endless output of soaps and odor-masking concoctions.
Sebaceous glands (Figure 30-5) are intimately associated with hair follicles, although some are free and open directly onto the surface. The cellular lining of the gland is discharged in the secretory process and must be renewed for further secretion. These gland cells become distended with a fatty accumulation, then die, and are expelled as a greasy mixture called sebum into the hair follicle. Called a “polite fat” because it does not turn rancid, it serves as a dressing to keep skin and hair pliable and glossy. Most mammals have sebaceous glands over the entire body; in humans they are most numerous in the scalp and on the face.
Mammary glands, which provide the name for mammals, are probably modified apocrine glands. Whatever their evolutionary origin, they occur on all female mammals and in a rudimentary form on all male mammals. They develop by the thickening of the epidermis to form a milk line along each side of the abdomen in the embryo. On certain parts of these lines the mammae appear while the intervening parts of the ridge disappear. Human female mammary glands begin to increase in size at puberty because of fat accumulation and reach their maximum development in approximately the twentieth year. Breasts (or mammae) undergo additional development during pregnancy. In other mammals the mammae are swollen only periodically when they are distended with milk during pregnancy and subsequent nursing of the young.
Food and Feeding
Mammals exploit an enormous variety of food sources; some mammals require highly specialized diets, whereas others are opportunistic feeders that thrive on diversified diets. Food habits and physical structure are thus inextricably linked. A mammal’s adaptations for attack and defense and its specializations for finding, capturing, chewing, swallowing, and digesting food all determine a mammal’s shape and habits.
Teeth, perhaps more than any other single physical characteristic, reveal the life habit of a mammal (Figure 30-10). With certain exceptions (monotremes, anteaters, certain whales), all mammals have teeth, except monotremes, anteaters, and certain whales, and their modifications are correlated with what the mammal eats.
As mammals evolved during the Mesozoic, major changes occurred in teeth and jaws. Unlike the uniform homodont dentition of the reptiles, mammalian teeth became differentiated to perform specialized functions such as cutting, seizing, gnawing, tearing, grinding, and chewing. Teeth differentiated in this manner are called heterodont. Mammalian dentition is differentiated into four types: incisors, with simple crowns and sharp edges, used mainly for snipping or biting; canines, with long conical crowns, specialized for piercing; premolars, with compressed crowns and one or two cusps, suited for shearing and slicing; and molars, with large bodies and variable cusp arrangement, used for crushing and grinding. The primitive tooth formula for most mammals, which expresses the number of each tooth type in one-half of the upper and lower jaw, was I 3/3, C 1/1, PM 4/4, M 3/3 = 44. Members of the order Insectivora (shrews), some omnivores, and carnivores come closest to this primitive pattern (Figure 30-10).
Unlike reptiles, mammals do not continuously replace their teeth throughout their lives. Most mammals grow just two sets of teeth: a temporary set, called deciduous, or milk, teeth, which is replaced by a permanent set when the skull has grown large enough to accommodate a full set. Only the incisors, canines, and premolars are deciduous; the molars are never replaced and the single permanent set must last a lifetime.
Feeding Specializations
The feeding, or trophic, apparatus of a mammal—teeth and jaws, tongue, and alimentary canal—are adapted to its particular feeding habits. Mammals are customarily divided among four basic categories—insectivores, carnivores, omnivores, and herbivores—but many other feeding specializations have evolved in mammals, as in other living organisms, and the feeding habits of many mammals defy exact classification. The principal feeding specializations of mammals are shown in Figure 30-10.
Insectivores are small mammals such as shrews, moles, anteaters, and most bats. They feed on insects, as well as a variety of small invertebrates, such as worms and grubs. Since insectivores eat little fibrous vegetable matter that requires prolonged fermentation, their intestinal tract tends to be short (Figure 30-11). The insectivorous category is not a sharply distinguished one because carnivores and omnivores may include insects in their diets. Even many rodents, which are considered herbivores, may have a mixed diet of insect larvae, seeds, and fruits.
Herbivorous mammals that feed on grasses and other vegetation form two main groups: browsers and grazers, such as the ungulates (hooved mammals including horses, deer, antelope, cattle, sheep, and goats), and the gnawers, such as the rodents, and rabbits and hares. In herbivores, the canines are absent or reduced in size, whereas the molars, which are adapted for grinding, are broad and usually high-crowned. Rodents (for example, beavers) have chisel-sharp incisors that grow throughout life and must be worn away to keep pace with their continual growth (Figure 30-10).
Herbivorous mammals have a number of interesting adaptations for dealing with their fibrous diet of plant food. Cellulose, the structural carbohydrate of plants, is composed of long chains of glucose molecules, and therefore is a potentially nutritious food resource. However, the glucose molecules in cellulose are linked by a type of chemical bond that few enzymes can attack. No vertebrates synthesize cellulose-splitting enzymes. Instead, herbivorous vertebrates harbor anaerobic bacteria and protozoa in huge fermentation chambers in their gut. These microorganisms break down and metabolize cellulose, releasing a variety of fatty acids, sugars, and starches that the host animal can absorb and utilize.
Some herbivores, such as horses, zebras, rabbits, hares, elephants, and many rodents, have a gut with a spacious sidepocket, or diverticulum, called a cecum, which serves as a fermentation chamber and absorptive area (Figure 30-11). Hares, rabbits, and some rodents often eat their fecal pellets (coprophagy), giving the food a second pass through the fermenting action of the intestinal microorganisms.
Ruminants (cattle, bison, buffalo, goats, antelopes, sheep, deer, giraffes, and okapis) have a huge four-chambered stomach (Figure 30-11). As a ruminant feeds, grass passes down the esophagus to the rumen, where it is broken down by microorganisms and then formed into small balls of cud. At its leisure, the ruminant returns the cud to its mouth where the cud is deliberately chewed at length to crush the fiber. Swallowed again, the food returns to the rumen where it is digested by the cellulolytic microorganisms. The pulp passes to the reticulum, then to the omasum, where water, soluble food, and microbial products are absorbed. The remainder proceeds to the abomasum (“true” acid stomach), where proteolytic enzymes are secreted and normal digestion takes place.
Herbivores generally have large, long digestive tracts and must eat a considerable amount of plant food to survive. An African elephant weighing 6 tons must consume 135 to 150 kg (300 to 400 pounds) of rough fodder each day to obtain sufficient nourishment for life.
Carnivorous mammals feed mainly on herbivores. This group includes foxes, dogs, weasels, wolverines, fishers, cats, lions, and tigers. Carnivores are well-equipped with biting and piercing teeth and powerful clawed limbs for killing their prey. Since their protein diet is more easily digested than the woody food of herbivores, their digestive tract is shorter and the cecum small or absent (Figure 30-11). Carnivores organize their feeding into discrete meals rather than feeding continuously (as do most herbivores) and therefore have much more leisure time.
In general, carnivores lead more active—and by human standards more interesting—lives than do the herbivores. Since a carnivore must find and catch its prey, there is a premium on intelligence; many carnivores, such as the cats, are noted for their stealth and cunning in hunting prey (Figure 30-12). This has led to a selection of herbivores capable either of defending themselves or of detecting and escaping carnivores. Thus for herbivores, there has been a premium on keen senses, speed and agility. Some herbivores, however, survive by virtue of their sheer size (rhinos, elephants) or by defensive group behavior (for example, muskoxen).
Humans have changed the rules in the carnivore-herbivore contest. Carnivores, despite their intelligence, have suffered much from human presence and have been virtually exterminated in some areas. Small herbivores, on the other hand, with their potent reproductive ability, have consistently defeated our most ingenious efforts to banish them from our environment. The problem of rodent pests in agriculture has intensified (Figure 30-29); we have removed carnivores, which served as the herbivores’ natural population control, but have not been able to devise a suitable substitute.
Omnivorous mammals—pigs, raccoons, rats, bears, and most primates, including humans—live on both plants and animals for food. Many carnivorous forms also eat fruits, berries, and grasses when hard pressed. Foxes, which usually feed on mice, small rodents, and birds, eat frozen apples, beechnuts, and corn when their normal food sources are scarce.
For most mammals, searching for food and eating occupy the majority of their active life. Seasonal changes in food supplies are considerable in temperate zones. Living may be easy in the summer when food is abundant, but in winter many carnivores must range far and wide to eke out a narrow existence. Some migrate to regions where food is more abundant while others hibernate and sleep the winter months away.
Many mammals cache food stores during periods of plenty. This habit is most pronounced in rodents, such as squirrels, chipmunks, gophers, and certain mice. All tree squirrels—red, fox, and gray—collect nuts, conifer seeds, and fungi and store these in caches for winter use. Often each item is hidden in a different place (scatter hoarding) and marked by a scent to assist relocation in the future. Some of the caches of chipmunks and red squirrels can be quite large (Figure 30-13).
Body Weight and Food Consumption
The relationship between body size and metabolic rate was discussed in relation to food consumption of birds. The smaller the animal, the greater is its metabolic rate and the more it must consume relative to its body size (Figure 30-14). This happens because the metabolic rate of an animal—and therefore the amount of food it must eat to sustain this metabolic rate—varies in rough proportion to the relative surface area rather than to the body weight. Surface area is proportional to approximately 0.7 power of body weight, and the amount of food a mammal (or bird) eats also is roughly proportional to a 0.7 power of its body weight. For example, a 3 g mouse will consume per gram body weight five times more food than does a 10 kg dog and about 30 times more food than does a 50,000 kg elephant. Thus small mammals (shrews, bats, and mice) must spend much more time hunting and eating food than do large mammals. The smallest shrews weighing only 2 g may eat more than their body weight each day and will starve to death in a few hours if deprived of food (Figure 30-15). In contrast, large carnivores can remain fat and healthy with only one meal every few days. Mountain lions are known to kill an average of one deer a week, although they will kill more frequently when game is abundant.
Migration
Migration is a more difficult undertaking for mammals than for birds. Not surprisingly, few mammals make regular seasonal migrations, preferring instead to center their activities in a defined and limited home range. Nevertheless, there are some striking examples of mammalian migrations. More migrators are found in North America than on any other continent.
An example is the barren-ground caribou of Canada and Alaska, which undertakes direct and purposeful mass migrations spanning 160 to 1100 km (100 to 700 miles) twice annually (Figure 30-16). From winter ranges in boreal forests (taiga), they migrate rapidly in late winter and spring to calving ranges on the barren grounds (tundra). The calves are born in mid- June. As summer progresses, caribou are increasingly harassed by warble and nostril flies that bore into their flesh, by mosquitoes that drink their blood (estimated at a liter per caribou each week during the height of the mosquito season), and by wolves that prey on their calves. They move southward in July and August, feeding little along the way. In September they reach the taiga and feed there almost continuously on low ground vegetation. Mating (rut) occurs in October.
The plains bison, before its deliberate near extinction by humans, made huge circular migrations to separate summer and winter ranges.
The longest mammalian migrations are made by the oceanic seals and whales. Gray whales, for example, migrate between Alaska in summer and Baja California, Mexico, in winter, an annual migration of over 18,000 km (11,250 miles). One of the most remarkable migrations is that of the fur seal, which breeds on the Pribilof Islands approximately 300 km (185 miles) off the coast of Alaska and north of the Aleutian Islands. From wintering grounds off southern California females journey as much as 2800 km (1740 miles) across open ocean, arriving in the spring at the Pribilofs where they congregate in enormous numbers (Figure 30-17). Young are born within a few hours or days after arrival of the cows. Then the bulls, having already arrived and established territories, collect harems of cows, which they guard with vigilance. After the calves have been nursed for approximately three months, cows and juveniles leave for their long migration southward. Bulls do not follow but remain in the Gulf of Alaska during the winter.
Although we might expect bats, the only winged mammals, to use their gift of flight to migrate, few of them do. Most spend winters in hibernation. Four species of American bats that migrate spend their summers in northern or western states and their winters in the southern United States or Mexico.
Flight and Echolocation
Mammals have not exploited the skies to the same extent that they have terrestrial and aquatic environments. However, many mammals scamper about in trees with amazing agility; some can glide from tree to tree (Figure 30-18) and one group, the bats, is capable of full flight. Gliding and flying evolved independently in several groups of mammals, including marsupials, rodents, flying lemurs, and bats. Anyone who has watched a gibbon perform in a zoo realizes there is something akin to flight in this primate, too. Among the arboreal squirrels, all of which are nimble acrobats, by far the most efficient is the flying squirrel (Figure 30-18). These forms actually glide rather than fly, using the gliding skin that extends from the sides of the body.
Bats are nocturnal or crepuscular (active at twilight) and thus hold a niche unoccupied by most birds. Their achievement is attributed to two attributes: flight and capacity to navigate by echolocation. Together these adaptations enable bats to fly and avoid obstacles in absolute darkness, to locate and catch insects with precision, and to find their way deep into caves (a habitat largely ignored by both mammals and birds) where they sleep during the daytime hours.
Research has been concentrated on members of the family Vespertilionidae, to which most of the common North American bats belong. When in flight, bats emit short pulses 5 to 10 msec in duration in a narrow directed beam from the mouth or nose (Figure 30-19). Each pulse is frequency modulated; that is, it is highest at the beginning, up to 100,000 Hz (hertz, cycles per second), and sweeps down to perhaps 30,000 Hz at the end. Sounds of this frequency are ultrasonic to human ears which have an upper limit of about 20,000 Hz. When bats are searching for prey, they produce about 10 pulses per second. If prey is detected, the rate increases rapidly up to 200 pulses per second in the final phase of approach and capture. Pulses are spaced so the echo of each is received before the next pulse is emitted, an adaptation that prevents jamming. Since transmission-to-reception time decreases as the bat approaches an object, it can increase the pulse frequency to obtain more information about the object. Pulse length is also shortened as the bat nears the object. It is interesting that some prey of bats, certain nocturnal moths for example, have evolved ultrasonic detectors used to detect and avoid approaching bats.
External ears of bats are large, like hearing trumpets, and shaped variously in different species. Less is known about the inner ear of bats, but it obviously is capable of receiving the ultrasonic sounds emitted. Biologists believe bat navigation is so refined that the bat builds up a mental image of its surroundings from echo scanning that approaches the resolution of a visual image from eyes of diurnal animals.
For reasons not fully understood, all bats are nocturnal, even the fruiteating bats that use vision and olfaction instead of sonar to find their food. The tropics and subtropics have many nectar-feeding bats that are important pollinators for a wide variety of chiropterophilous (“batloving”) plants. Flowers of these plants open at night, are white or light in color, and emit a musky, batlike odor that nectar-feeding bats find attractive.
The famed tropical vampire bat has razor-sharp incisors used to shave away the epidermis of its prey, exposing underlying capillaries. After infusing an anticoagulant to aid blood flow, it laps up and stores its meal in a specially modified stomach.
Reproduction
Reproductive Cycles
Most mammals have definite mating seasons, usually in winter or spring and timed to coincide with the most favorable time of the year for rearing young after birth. Many male mammals are capable of fertile copulation at any time, but female mating function is restricted to a time during a periodic cycle, known as the estrous cycle. Females only copulate with males during a relatively brief period known as heat or estrus. (Figure 30-20).
The estrous cycle is divided into stages marked by characteristic changes in the ovaries, uterus, and vagina. Proestrus, or period of preparation, when new ovarian follicles grow, is followed by estrus, when mating occurs. Almost simultaneously ovarian follicles burst, releasing eggs (ovulation), which are fertilized. In all placental mammals, the fertilized egg implants itself in the uterine wall with pregnancy following. However, should mating and fertilization not occur, estrus is followed by metestrus, a period of repair. This stage is followed by diestrus, during which the uterus becomes small and anemic. The cycle then repeats itself, beginning with proestrus.
How often females are in estrus varies greatly among different mammals. Animals that have only a single estrus during their breeding season are called monestrous; those that have a recurrence of estrus during their breeding season are called polyestrous. Dogs, foxes, and bats belong to the first group; field mice and squirrels are all polyestrous as are many mammals living in the more tropical regions of the earth. Old World monkeys and humans have a somewhat different cycle in which the postovulation period is terminated by menstruation, during which the, endometrium (lining of the uterus) collapses and is discharged with some blood. This menstrual cycle is described in The Reproductive Process.
Reproductive Patterns
There are three different patterns of reproduction in mammals. One pattern is represented by egg-laying (oviparous) mammals, the monotremes. The duck-billed platypus has one breeding season each year. Ovulated eggs, usually two, are fertilized in the oviduct. As they continue down the oviduct, various glands add albumin and then a thin, leathery shell to each egg. When laid, the eggs are about the size of a robin’s egg. The platypus lays its eggs in a burrow nest where they are incubated for about 12 days. After hatching, the young suck milk from the fur of the mother around openings of the mammary glands. Thus in monotremes there is no gestation (period of pregnancy) and the developing embryos draw nutrients stored in their eggs, much as do the embryos of reptiles and birds. But in common with all other mammals, monotremes rear their young on milk.
Marsupials are pouched, viviparous mammals that exhibit a second pattern of reproduction. Although only the eutherians are called “placental mammals,” the marsupials do have a primitive type of placenta, called a choriovitelline (or yolk sac) placenta. The embryo (blastocyst) of a marsupial is at first encapsulated by shell membranes and floats free for several days in the uterine fluid. After “hatching” from the shell membranes, the embryo does not implant, or “take root” in the uterus as it would in eutherians, but it does erode a shallow depression in the uterine wall in which it lies and absorbs nutrient secretions from the mucosa by way of the vascularized yolk sac. Gestation (the intrauterine period of development) is brief in marsupials, and therefore all marsupials give birth to tiny young that are effectively still embryos, both anatomically and physiologically. However, early birth is followed by a prolonged interval of lactation and parental care (Figure 30-21).
In red kangaroos (Figure 30-22) the
first pregnancy of the season is followed
by a 33-day gestation, after
which the young (joey) is born, crawls
to the pouch without assistance from
the mother, and attaches to a nipple.
The mother immediately becomes
pregnant again, but the presence of a
suckling young in the pouch arrests
development of the new embryo in the
uterus at about the 100-cell stage. This
period of arrest, called embryonic diapause,
lasts approximately 235 days
during which time the first joey is growing
in the pouch. When the joey leaves
the pouch, the uterine embryo resumes
development and is born about a
month later. The mother again becomes
pregnant, but because the second joey
is suckling, once again development of
the new embryo is arrested. Meanwhile,
the first joey returns to the pouch
from time to time to suckle. At this
point the mother has three young of
different ages dependent upon her for
nourishment: a joey on foot, a joey in
the pouch, and a diapause embryo in
the uterus. There are variations on this
remarkable sequence—not all marsupials
have developmental delays like kangaroos,
and some do not even have
pouches—but in all, the young are
born at an extremely early stage of
development and undergo prolonged
development while dependent on a
teat (Figure 30-23).
The third pattern of reproduction is that of viviparous placental mammals, the eutherians. In placentals, the reproductive investment is in prolonged gestation, unlike marsupials in which the reproductive investment is in prolonged lactation (Figure 30-21). The embryo remains in the uterus, nourished by food supplied through a chorioallantoic type of placenta, an intimate connection between mother and young. Length of gestation is longer in placentals than marsupials, and in large mammals it is much longer (Figure 30- 21). For example, mice have a gestation period of 21 days; rabbits and hares, 30 to 36 days; cats and dogs, 60 days; cattle, 280 days; and elephants, 22 months (the longest). But there are important exceptions (nature seldom offers perfect correlations). Baleen whales, the largest mammals, carry their young for only 12 months, while bats, no larger than mice, have gestation periods of 4 to 5 months. The condition of the young at birth also varies. An antelope bears its young well furred, eyes open, and able to run about. Newborn mice, however, are blind, naked, and helpless. We all know how long it takes a human baby to gain its footing. Human growth is in fact slower than that of any other mammal, and this is one of the distinctive attributes that sets us apart from other mammals.
The number of young produced by mammals in a season depends on mortality rate, which, for some mammals such as mice, may be high at all age levels. Usually, the larger the animal, the smaller the number of young in a litter. Small rodents, which serve as prey for many carnivores, usually produce more than one litter of several young each season. Meadow mice are known to produce as many as 17 litters of four to nine young in a year. Most carnivores have but one litter of three to five young per year. Large mammals, such as elephants and horses, give birth to a single young with each pregnancy. An elephant produces, on average, four calves during her reproductive life of perhaps 50 years.
Territory and Home Range
Many mammals have territories—areas from which individuals of the same species are excluded. In fact, many wild mammals, like some humans are basically unfriendly to their own kind, especially so to their own sex during the breeding season. If the mammal dwells in a burrow or den, this area forms the center of its territory. If it has no fixed address, the territory is marked out, usually with the highly developed scent glands. Territories vary greatly in size depending on the size of the animal and its feeding habits. Grizzly bears have territories of several square miles, which they guard zealously against all other grizzlies.
Mammals usually use natural features of their surroundings in staking their claims. These are marked with secretions from the scent glands or by urinating or defecating. When an intruder knowingly enters another’s marked territory, it is immediately placed at a psychological disadvantage. Should a challenge follow, the intruder almost invariably breaks off the encounter in a submissive display characteristic for the species. Territoriality and aggressive and submissive displays are described in more detail in Animal Behavior.
A beaver colony is a family unit, and beavers are among several mammalian species in which the male and female form a strong monogamous bond that lasts a lifetime. Because beavers invest considerable time and energy in constructing a lodge and dam and storing food for winter (Figure 30-24), the family, especially the adult male, vigorously defends its real estate against intruding beavers. Most of the work of building dams and lodges is undertaken by male beavers, but the females assist when not occupied with their young.
An interesting exception to the strong territorial nature of most mammals is the prairie dog, which lives in large, friendly communities called prairie dog “towns” (Figure 30-25). When a new litter has been reared, adults relinquish the old home to the young and move to the edge of the community to establish a new home. Such a practice is totally antithetical to the behavior of most mammals, which drive off the young when they are self sufficient.
The home range of a mammal is a much larger foraging area surrounding a defended territory. Home ranges are not defended in the same way as are territories; home ranges may, in fact, overlap, producing a neutral zone used by owners of several territories for seeking food.
Mammalian Populations
A population of animals includes all members of a species that share a particular space and potentially interbreed (Animal Ecology). All mammals (like other organisms) live in ecological communities, each composed of numerous populations of different animal and plant species. Each species is affected by the activities of other species and by other changes, especially climatic, that occur. Thus populations are always changing in size. Populations of small mammals are lowest before the breeding season and greatest just after the addition of new members. Beyond these expected changes in population size, mammalian populations may fluctuate from other causes.
Irregular fluctuations are commonly produced by variations in climate, such as unusually cold, hot, or dry weather, or by natural catastrophes, such as fires, hailstorms, and hurricanes. These are density-independent factors because they affect a population whether it is crowded or dispersed. However, the most spectacular fluctuations are density dependent; that is, they correlate with population crowding.
Cycles of abundance are common among many rodent species. One of the best-known examples is the mass migrations of the Scandinavian and arctic North American lemmings following population peaks. Lemmings (Figure 30-26) breed all year, although more in summer than in winter. The gestation period is only 21 days; young born at the beginning of the summer are weaned in 14 days and are capable of reproducing by end of summer. At the peak of their population density, having devastated the vegetation by tunneling and grazing, lemmings begin long, mass migrations to find new undamaged habitats for food and space. They swim across streams and small lakes as they go but cannot distinguish these from large lakes, rivers, and the sea, in which they drown. Since lemmings are the main diet of many carnivorous mammals and birds, any change in lemming population density affects all their predators as well.
Varying hares (snowshoe rabbits, Figure 30-7) of North America show 10- year cycles in abundance. The wellknown fecundity of rabbits enables them to produce litters of three or four young as many as five times per year. The density may increase to 4000 hares competing for food in each square mile of northern forest. Predators (owls, minks, foxes, and especially lynxes) also increase (Figure 30-27). Then the population crashes precipitously for reasons that have long been a puzzle to scientists. Rabbits die in great numbers, not from lack of food or from an epidemic disease (as was once believed) but evidently from some density-dependent psychogenic cause. As crowding increases, hares become more aggressive, show signs of fear and defense, and stop breeding. The entire population reveals symptoms of pituitary-adrenal gland exhaustion, an endocrine imbalance called “shock disease,” which results in death. These dramatic crashes are not well understood. Whatever the causes, population crashes that follow superabundance, although harsh, permit the vegetation to recover, providing survivors with a much better chance for successful breeding.
Integument and Its Derivatives
Mammalian skin and especially its modifications distinguish mammals as a group. As the interface between an animal and its environment, the skin is strongly molded by the animal’s way of life. In general the skin is thicker in mammals than in other classes of vertebrates, although as in all vertebrates it is made up of epidermis and dermis (see Figure 30-5). Among mammals the dermis becomes much thicker than the epidermis. The epidermis is thinner where it is well protected by hair, but in places that are subject to much contact and use, such as the palms or soles, its outer layers become thick and cornified with keratin.
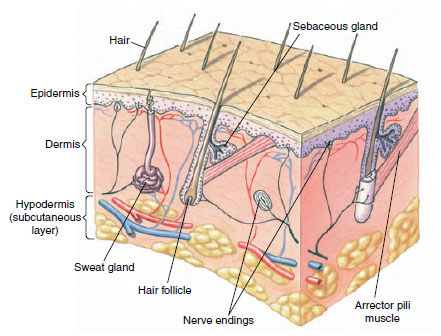 |
| Figure 30-5 Structure of human skin (epidermis and dermis) and hypodermis, showing hair and glands. |
Hair
Hair is especially characteristic of mammals, although humans are not very hairy creatures and, in whales, hair is reduced to only a few sensory bristles on the snout. A hair grows from a hair follicle that, although an epidermal structure, is sunk into the dermis of the skin (Figure 30-5). The hair grows continuously by rapid proliferation of cells in the follicle. As the hair shaft is pushed upward, new cells are carried away from their source of nourishment and die, turning into the same dense type of fibrous protein, called keratin, that constitutes nails, claws, hooves, and feathers.
Mammals characteristically have two kinds of hair forming the pelage (fur coat): (1) dense and soft under hair for insulation and (2) coarse and longer guard hair for protection against wear and to provide coloration. Underhair traps a layer of insulating air. In aquatic mammals, such as fur seals, otters, and beavers, it is so dense that it is almost impossible to wet. In water, guard hairs become wet and mat down, forming a protective blanket over the underhair (Figure 30-6).
 |
| Figure 30-6 American beaver, Castor canadensis, gnawing on an aspen tree. This second largest rodent (the South American capybara is larger) has a heavy waterproof pelage consisting of long, tough guard hairs overlying the thick, silky underhair so valued in the fur trade. Order Rodentia, family Castoridae. |
 |
| Figure 30-7 Snowshoe, or varying, hare, Lepus americanus in A, brown summer coat and, in B, white winter coat. In winter, extra hair growth on the hind feet broadens the animal’s support in snow. Snowshoe hares are common residents of the taiga and are an important prey for lynxes, foxes, and other carnivores. Population fluctuations of hares and their predators are closely related. Order Lagomorpha family Leporidae. |
In the simplest cases, such as foxes and seals, the coat is shed once every summer. Most mammals have two annual molts, one in the spring and one in the fall. Summer coats are always much thinner than winter coats and in some mammals it may be a different color. Several northern mustelid carnivores, for example, weasels, have white winter coats and brown-colored summer coats. It was once believed that the white inner pelage of arctic animals conserves body heat by reducing radiation loss; in fact, dark and white pelages radiate heat equally well. Winter white pelage of arctic animals is simply camouflage in a land of snow. The varying hare of North America has three annual molts: the white winter coat is replaced by a brownish gray summer coat, and this is replaced in autumn by a grayer coat, which is soon shed to reveal the winter white coat beneath (Figure 30-7). White fur of arctic mammals in winter (leukemism) is not to be confused with albinism, caused by a recessive gene that blocks pigment formation. Albinos have red eyes and pinkish skin, whereas arctic animals in their winter coats have dark eyes and often dark-colored ear tips, noses, and tail tips.
Outside the Arctic, most mammals wear somber colors that are protective. Often the species is marked with “saltand- pepper” coloration or a disruptive pattern that helps make it inconspicuous in its natural surroundings. Examples are the spots of leopards and fawns and the stripes of tigers. Skunks advertise their presence with conspicuous warning coloration.
The hair of mammals has become modified to serve many purposes. Bristles of hogs, spines of porcupines and their kin, and vibrissae on the snouts of most mammals are examples. Vibrissae, commonly called “whiskers,” are really sensory hairs that provide a tactile sense to many mammals. The slightest movement of a vibrissa generates impulses in sensory nerve endings that travel to special sensory areas in the brain. Vibrissae are especially long in nocturnal and burrowing animals.
 |
| Figure 30-8 Dogs are frequent victims of the porcupine’s impressive quills. Unless removed (usually by a veterinarian) quills will continue to work their way deeper in the flesh causing great distress and may lead to the victim’s death. |
Porcupines, hedgehogs, echidnas, and a few other mammals have developed an effective and dangerous spiny armor. When cornered, the common North American porcupine turns its back toward the attacker and lashes out with the barbed tail. The lightlyattached quills break off at their bases when they enter the skin and, aided by backward-pointed hooks on the tips, work deeply into tissues. Dogs are frequent victims (Figure 30-8) but fishers, wolverines, and bobcats are able to flip the porcupine onto its back to expose vulnerable underparts.
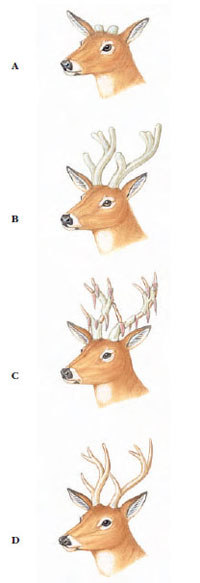 |
| Figure 30-9 Annual growth of buck deer antlers. A, Antlers begin growth in late spring, stimulated by pituitary gonadotropins. B, Bone grows very rapidly until halted by a rapid rise in testosterone production by the testes. C, The skin (velvet) dies and sloughs off. D, Testosterone levels peak during the fall breeding season. Antlers are shed in January as testosterone levels subside. |
Horns and Antlers
Three kinds of horns or hornlike structures are found in mammals. True horns, found in ruminants (for example, sheep and cattle), are hollow sheaths of keratinized epidermis that embrace a core of bone arising from the skull. True horns are not normally shed, usually are not branched (although they may be greatly curved), grow continuously, and are found in both sexes. Horns may be absent from pronghorn antelope females but, if present, are shorter than those of the male.
Antlers of the deer family are branched and composed of solid bone when mature. During their annual spring growth, antlers develop beneath a covering of highly vascular soft skin called velvet (Figure 30-9). Except for caribou (Figure 30-16A), only males of the species produce antlers. When growth of the antlers is complete just before the fall breeding season, the blood vessels constrict and the stag removes the velvet by rubbing the antlers against trees. Antlers are shed after the breeding season. New buds appear a few months later to herald the next set of antlers. For several years each new pair of antlers is larger and more elaborate than the previous set. Annual growth of antlers places a strain on the mineral metabolism, since during the growing season an older moose or elk must accumulate 50 or more pounds of calcium salts from its vegetable diet.
The rhinoceros horn is the third kind of hornlike structure. Hairlike keratinized filaments that arise from dermal papillae are cemented together to form these structures, which are not attached to the skull.
Glands
Of all vertebrates, mammals have the greatest variety of integumentary glands. Most fall into one of four classes: sweat, scent, sebaceous, and mammary. All are derivatives of the epidermis (Figure 30-5).
Sweat glands are tubular, highly coiled glands that occur over much of the body surface in most mammals (Figure 30-5). They are not present in other vertebrates. There are two kinds of sweat glands: eccrine and apocrine. Eccrine glands secrete a watery fluid that, if evaporated on the skin’s surface, draws heat away from the skin and cools it. Eccrine glands occur in hairless regions, especially the foot pads, in most mammals, although in horses and most primates they are scattered over the body. They are either reduced or absent in rodents, rabbits, and whales. Apocrine glands are larger than eccrine glands and have longer and more convoluted ducts. Their secretory coil is in the dermis and extends deep into the hypodermis. They always open into a hair follicle or where a hair once was. Apocrine gland development occurs near puberty and is restricted (in the human species) to the axillae (armpits), mons pubis, breasts, prepuce, scrotum, and external auditory canals. In contrast to the watery secretions of eccrine glands, apocrine secretions are milky fluids, whitish or yellow in color, that dry on the skin to form a film. Apocrine glands are not involved in heat regulation. Their activity is correlated with certain aspects of the reproductive cycle.
Scent glands are present in nearly all mammals. Their location and functions vary greatly. They are used for communication with members of the same species, for marking territorial boundaries, for warning, or for defense. Scent-producing glands are located in orbital, metatarsal, and inter-digital regions (deer); behind the eyes and on the cheek (pica and woodchuck); penis (muskrats, beavers, and many canines); base of the tail (wolves and foxes); back of the head (dromedary); and anal region (skunks, minks, and weasels). The latter, the most odoriferous of all glands, open by ducts into the anus; their secretions can be discharged forcefully for 2 to 3 meters. During the mating season many mammals give off strong scents for attracting the opposite sex. Humans also are endowed with scent glands. However civilization has taught us to dislike our own scent, a concern that has stimulated a lucrative deodorant industry to produce an endless output of soaps and odor-masking concoctions.
Sebaceous glands (Figure 30-5) are intimately associated with hair follicles, although some are free and open directly onto the surface. The cellular lining of the gland is discharged in the secretory process and must be renewed for further secretion. These gland cells become distended with a fatty accumulation, then die, and are expelled as a greasy mixture called sebum into the hair follicle. Called a “polite fat” because it does not turn rancid, it serves as a dressing to keep skin and hair pliable and glossy. Most mammals have sebaceous glands over the entire body; in humans they are most numerous in the scalp and on the face.
Mammary glands, which provide the name for mammals, are probably modified apocrine glands. Whatever their evolutionary origin, they occur on all female mammals and in a rudimentary form on all male mammals. They develop by the thickening of the epidermis to form a milk line along each side of the abdomen in the embryo. On certain parts of these lines the mammae appear while the intervening parts of the ridge disappear. Human female mammary glands begin to increase in size at puberty because of fat accumulation and reach their maximum development in approximately the twentieth year. Breasts (or mammae) undergo additional development during pregnancy. In other mammals the mammae are swollen only periodically when they are distended with milk during pregnancy and subsequent nursing of the young.
Food and Feeding
Mammals exploit an enormous variety of food sources; some mammals require highly specialized diets, whereas others are opportunistic feeders that thrive on diversified diets. Food habits and physical structure are thus inextricably linked. A mammal’s adaptations for attack and defense and its specializations for finding, capturing, chewing, swallowing, and digesting food all determine a mammal’s shape and habits.
Teeth, perhaps more than any other single physical characteristic, reveal the life habit of a mammal (Figure 30-10). With certain exceptions (monotremes, anteaters, certain whales), all mammals have teeth, except monotremes, anteaters, and certain whales, and their modifications are correlated with what the mammal eats.
As mammals evolved during the Mesozoic, major changes occurred in teeth and jaws. Unlike the uniform homodont dentition of the reptiles, mammalian teeth became differentiated to perform specialized functions such as cutting, seizing, gnawing, tearing, grinding, and chewing. Teeth differentiated in this manner are called heterodont. Mammalian dentition is differentiated into four types: incisors, with simple crowns and sharp edges, used mainly for snipping or biting; canines, with long conical crowns, specialized for piercing; premolars, with compressed crowns and one or two cusps, suited for shearing and slicing; and molars, with large bodies and variable cusp arrangement, used for crushing and grinding. The primitive tooth formula for most mammals, which expresses the number of each tooth type in one-half of the upper and lower jaw, was I 3/3, C 1/1, PM 4/4, M 3/3 = 44. Members of the order Insectivora (shrews), some omnivores, and carnivores come closest to this primitive pattern (Figure 30-10).
Unlike reptiles, mammals do not continuously replace their teeth throughout their lives. Most mammals grow just two sets of teeth: a temporary set, called deciduous, or milk, teeth, which is replaced by a permanent set when the skull has grown large enough to accommodate a full set. Only the incisors, canines, and premolars are deciduous; the molars are never replaced and the single permanent set must last a lifetime.
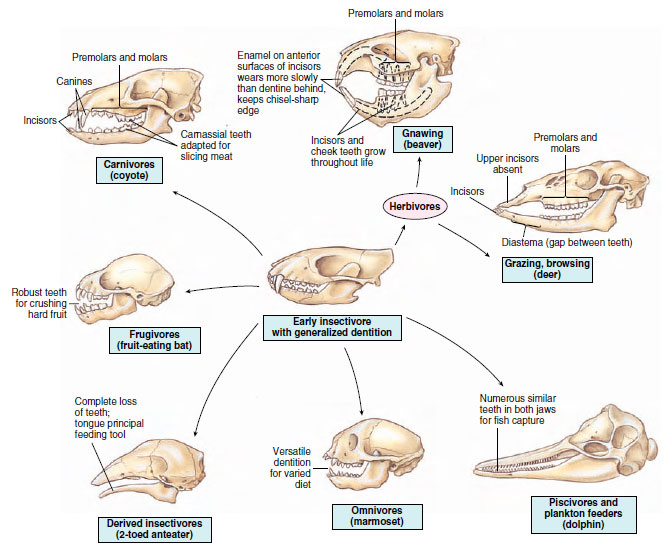 |
| Figure 30-10 Feeding specializations of major trophic groups of eutherian mammals. Early eutherians were insectivores; all other types are descended from them. |
Feeding Specializations
The feeding, or trophic, apparatus of a mammal—teeth and jaws, tongue, and alimentary canal—are adapted to its particular feeding habits. Mammals are customarily divided among four basic categories—insectivores, carnivores, omnivores, and herbivores—but many other feeding specializations have evolved in mammals, as in other living organisms, and the feeding habits of many mammals defy exact classification. The principal feeding specializations of mammals are shown in Figure 30-10.
Insectivores are small mammals such as shrews, moles, anteaters, and most bats. They feed on insects, as well as a variety of small invertebrates, such as worms and grubs. Since insectivores eat little fibrous vegetable matter that requires prolonged fermentation, their intestinal tract tends to be short (Figure 30-11). The insectivorous category is not a sharply distinguished one because carnivores and omnivores may include insects in their diets. Even many rodents, which are considered herbivores, may have a mixed diet of insect larvae, seeds, and fruits.
Herbivorous mammals that feed on grasses and other vegetation form two main groups: browsers and grazers, such as the ungulates (hooved mammals including horses, deer, antelope, cattle, sheep, and goats), and the gnawers, such as the rodents, and rabbits and hares. In herbivores, the canines are absent or reduced in size, whereas the molars, which are adapted for grinding, are broad and usually high-crowned. Rodents (for example, beavers) have chisel-sharp incisors that grow throughout life and must be worn away to keep pace with their continual growth (Figure 30-10).
Herbivorous mammals have a number of interesting adaptations for dealing with their fibrous diet of plant food. Cellulose, the structural carbohydrate of plants, is composed of long chains of glucose molecules, and therefore is a potentially nutritious food resource. However, the glucose molecules in cellulose are linked by a type of chemical bond that few enzymes can attack. No vertebrates synthesize cellulose-splitting enzymes. Instead, herbivorous vertebrates harbor anaerobic bacteria and protozoa in huge fermentation chambers in their gut. These microorganisms break down and metabolize cellulose, releasing a variety of fatty acids, sugars, and starches that the host animal can absorb and utilize.
Some herbivores, such as horses, zebras, rabbits, hares, elephants, and many rodents, have a gut with a spacious sidepocket, or diverticulum, called a cecum, which serves as a fermentation chamber and absorptive area (Figure 30-11). Hares, rabbits, and some rodents often eat their fecal pellets (coprophagy), giving the food a second pass through the fermenting action of the intestinal microorganisms.
Ruminants (cattle, bison, buffalo, goats, antelopes, sheep, deer, giraffes, and okapis) have a huge four-chambered stomach (Figure 30-11). As a ruminant feeds, grass passes down the esophagus to the rumen, where it is broken down by microorganisms and then formed into small balls of cud. At its leisure, the ruminant returns the cud to its mouth where the cud is deliberately chewed at length to crush the fiber. Swallowed again, the food returns to the rumen where it is digested by the cellulolytic microorganisms. The pulp passes to the reticulum, then to the omasum, where water, soluble food, and microbial products are absorbed. The remainder proceeds to the abomasum (“true” acid stomach), where proteolytic enzymes are secreted and normal digestion takes place.
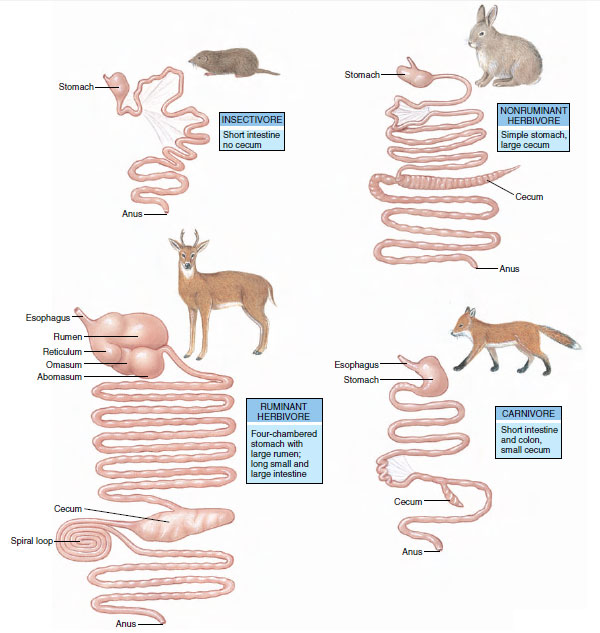 |
| Figure 30-11 Digestive systems of mammals, showing different morphology with different diets. |
Herbivores generally have large, long digestive tracts and must eat a considerable amount of plant food to survive. An African elephant weighing 6 tons must consume 135 to 150 kg (300 to 400 pounds) of rough fodder each day to obtain sufficient nourishment for life.
Carnivorous mammals feed mainly on herbivores. This group includes foxes, dogs, weasels, wolverines, fishers, cats, lions, and tigers. Carnivores are well-equipped with biting and piercing teeth and powerful clawed limbs for killing their prey. Since their protein diet is more easily digested than the woody food of herbivores, their digestive tract is shorter and the cecum small or absent (Figure 30-11). Carnivores organize their feeding into discrete meals rather than feeding continuously (as do most herbivores) and therefore have much more leisure time.
 |
| Figure 30-12 Lionesses, Panthera leo, eating a wildebeest. Lacking stamina for a long chase, lions stalk prey and then charge suddenly surprising the prey. Lions gorge themselves with their kill, then sleep and rest for periods as long as one week before eating again. Order Carnivora, family Felidae. |
In general, carnivores lead more active—and by human standards more interesting—lives than do the herbivores. Since a carnivore must find and catch its prey, there is a premium on intelligence; many carnivores, such as the cats, are noted for their stealth and cunning in hunting prey (Figure 30-12). This has led to a selection of herbivores capable either of defending themselves or of detecting and escaping carnivores. Thus for herbivores, there has been a premium on keen senses, speed and agility. Some herbivores, however, survive by virtue of their sheer size (rhinos, elephants) or by defensive group behavior (for example, muskoxen).
Humans have changed the rules in the carnivore-herbivore contest. Carnivores, despite their intelligence, have suffered much from human presence and have been virtually exterminated in some areas. Small herbivores, on the other hand, with their potent reproductive ability, have consistently defeated our most ingenious efforts to banish them from our environment. The problem of rodent pests in agriculture has intensified (Figure 30-29); we have removed carnivores, which served as the herbivores’ natural population control, but have not been able to devise a suitable substitute.
Omnivorous mammals—pigs, raccoons, rats, bears, and most primates, including humans—live on both plants and animals for food. Many carnivorous forms also eat fruits, berries, and grasses when hard pressed. Foxes, which usually feed on mice, small rodents, and birds, eat frozen apples, beechnuts, and corn when their normal food sources are scarce.
 |
| Figure 30-13 Eastern chipmunk, Tamias striatus, with cheek pouches stuffed with seeds to be carried to a hidden cache. It will try to store several liters of food for the winter. It hibernates but awakens periodically to eat some of its cached food. Order Rodentia, family Sciuridae. |
For most mammals, searching for food and eating occupy the majority of their active life. Seasonal changes in food supplies are considerable in temperate zones. Living may be easy in the summer when food is abundant, but in winter many carnivores must range far and wide to eke out a narrow existence. Some migrate to regions where food is more abundant while others hibernate and sleep the winter months away.
Many mammals cache food stores during periods of plenty. This habit is most pronounced in rodents, such as squirrels, chipmunks, gophers, and certain mice. All tree squirrels—red, fox, and gray—collect nuts, conifer seeds, and fungi and store these in caches for winter use. Often each item is hidden in a different place (scatter hoarding) and marked by a scent to assist relocation in the future. Some of the caches of chipmunks and red squirrels can be quite large (Figure 30-13).
Body Weight and Food Consumption
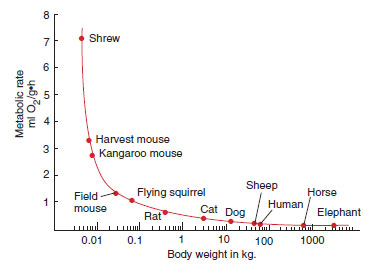
The relationship between body size and metabolic rate was discussed in relation to food consumption of birds. The smaller the animal, the greater is its metabolic rate and the more it must consume relative to its body size (Figure 30-14). This happens because the metabolic rate of an animal—and therefore the amount of food it must eat to sustain this metabolic rate—varies in rough proportion to the relative surface area rather than to the body weight. Surface area is proportional to approximately 0.7 power of body weight, and the amount of food a mammal (or bird) eats also is roughly proportional to a 0.7 power of its body weight. For example, a 3 g mouse will consume per gram body weight five times more food than does a 10 kg dog and about 30 times more food than does a 50,000 kg elephant. Thus small mammals (shrews, bats, and mice) must spend much more time hunting and eating food than do large mammals. The smallest shrews weighing only 2 g may eat more than their body weight each day and will starve to death in a few hours if deprived of food (Figure 30-15). In contrast, large carnivores can remain fat and healthy with only one meal every few days. Mountain lions are known to kill an average of one deer a week, although they will kill more frequently when game is abundant.
 |
 |
|
| Figure 30-14 Relationship between body weight and metabolic rate for mammals. This relationship often called the “mouse-to-elephant” curve, shows that metabolic rate is intense for small mammals like shrews and mice, and declines with increasing body weight of the species. |
Figure 30-15 The shorttail shrew, Blarina brevicauda, eating a grasshopper. This tiny but fierce mammal, with a prodigious appetite for insects, mice, snails, and worms, spends most of its time underground and so is seldom seen. Shrews are believed to resemble insectivorous ancestors of placental mammals. Order Insectivora, family Soricidae |
Migration
Migration is a more difficult undertaking for mammals than for birds. Not surprisingly, few mammals make regular seasonal migrations, preferring instead to center their activities in a defined and limited home range. Nevertheless, there are some striking examples of mammalian migrations. More migrators are found in North America than on any other continent.
An example is the barren-ground caribou of Canada and Alaska, which undertakes direct and purposeful mass migrations spanning 160 to 1100 km (100 to 700 miles) twice annually (Figure 30-16). From winter ranges in boreal forests (taiga), they migrate rapidly in late winter and spring to calving ranges on the barren grounds (tundra). The calves are born in mid- June. As summer progresses, caribou are increasingly harassed by warble and nostril flies that bore into their flesh, by mosquitoes that drink their blood (estimated at a liter per caribou each week during the height of the mosquito season), and by wolves that prey on their calves. They move southward in July and August, feeding little along the way. In September they reach the taiga and feed there almost continuously on low ground vegetation. Mating (rut) occurs in October.
The plains bison, before its deliberate near extinction by humans, made huge circular migrations to separate summer and winter ranges.
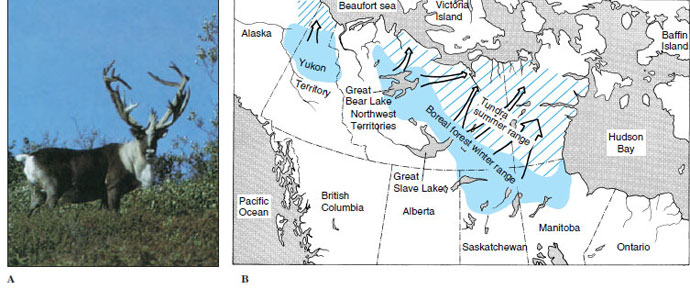 |
| Figure 30-16 Barren-ground caribou, Rangifer tarandus, of Canada and Alaska. A, Adult male caribou in autumn pelage and antlers in velvet. B, Summer and winter ranges of some major caribou herds in Canada and Alaska (other herds not shown occur on Baffin Island and in western and central Alaska). Principal spring migration routes are indicated by arrows; routes vary considerably from year to year. The same species is known as reindeer in Europe. Order Artiodactyla, family Cervidae. |
The longest mammalian migrations are made by the oceanic seals and whales. Gray whales, for example, migrate between Alaska in summer and Baja California, Mexico, in winter, an annual migration of over 18,000 km (11,250 miles). One of the most remarkable migrations is that of the fur seal, which breeds on the Pribilof Islands approximately 300 km (185 miles) off the coast of Alaska and north of the Aleutian Islands. From wintering grounds off southern California females journey as much as 2800 km (1740 miles) across open ocean, arriving in the spring at the Pribilofs where they congregate in enormous numbers (Figure 30-17). Young are born within a few hours or days after arrival of the cows. Then the bulls, having already arrived and established territories, collect harems of cows, which they guard with vigilance. After the calves have been nursed for approximately three months, cows and juveniles leave for their long migration southward. Bulls do not follow but remain in the Gulf of Alaska during the winter.
Although we might expect bats, the only winged mammals, to use their gift of flight to migrate, few of them do. Most spend winters in hibernation. Four species of American bats that migrate spend their summers in northern or western states and their winters in the southern United States or Mexico.
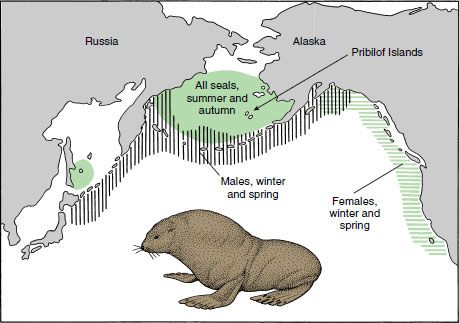 |
| Figure 30-17 Annual migrations of the northern fur seals, showing the separate wintering grounds of males and females. Both males and females of the larger Pribilof population migrate in early summer to the Pribilof Islands, where females give birth to their pups and then mate. Order Carnivora, family Otariidae. |
 |
| Figure 30-18 Eastern flying squirrel, Glaucomys sabrinus, gliding in for a landing. Area of undersurface is nearly trebled when gliding skin is spread. Glides of 40 to 50 m are possible. Good maneuverability during flight is achieved by adjusting position of the gliding skin with special muscles. Flying squirrels are nocturnal and have superb night vision. Order Rodentia, family Sciuridae. |
Flight and Echolocation
Mammals have not exploited the skies to the same extent that they have terrestrial and aquatic environments. However, many mammals scamper about in trees with amazing agility; some can glide from tree to tree (Figure 30-18) and one group, the bats, is capable of full flight. Gliding and flying evolved independently in several groups of mammals, including marsupials, rodents, flying lemurs, and bats. Anyone who has watched a gibbon perform in a zoo realizes there is something akin to flight in this primate, too. Among the arboreal squirrels, all of which are nimble acrobats, by far the most efficient is the flying squirrel (Figure 30-18). These forms actually glide rather than fly, using the gliding skin that extends from the sides of the body.
Bats are nocturnal or crepuscular (active at twilight) and thus hold a niche unoccupied by most birds. Their achievement is attributed to two attributes: flight and capacity to navigate by echolocation. Together these adaptations enable bats to fly and avoid obstacles in absolute darkness, to locate and catch insects with precision, and to find their way deep into caves (a habitat largely ignored by both mammals and birds) where they sleep during the daytime hours.
Research has been concentrated on members of the family Vespertilionidae, to which most of the common North American bats belong. When in flight, bats emit short pulses 5 to 10 msec in duration in a narrow directed beam from the mouth or nose (Figure 30-19). Each pulse is frequency modulated; that is, it is highest at the beginning, up to 100,000 Hz (hertz, cycles per second), and sweeps down to perhaps 30,000 Hz at the end. Sounds of this frequency are ultrasonic to human ears which have an upper limit of about 20,000 Hz. When bats are searching for prey, they produce about 10 pulses per second. If prey is detected, the rate increases rapidly up to 200 pulses per second in the final phase of approach and capture. Pulses are spaced so the echo of each is received before the next pulse is emitted, an adaptation that prevents jamming. Since transmission-to-reception time decreases as the bat approaches an object, it can increase the pulse frequency to obtain more information about the object. Pulse length is also shortened as the bat nears the object. It is interesting that some prey of bats, certain nocturnal moths for example, have evolved ultrasonic detectors used to detect and avoid approaching bats.
External ears of bats are large, like hearing trumpets, and shaped variously in different species. Less is known about the inner ear of bats, but it obviously is capable of receiving the ultrasonic sounds emitted. Biologists believe bat navigation is so refined that the bat builds up a mental image of its surroundings from echo scanning that approaches the resolution of a visual image from eyes of diurnal animals.
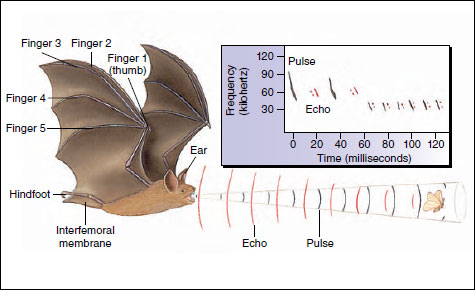 |
| Figure 30-19 Echolocation of an insect by the little brown bat Myotis lucifugus. Frequency modulated pulses are directed in a narrow beam from the bat’s mouth. As the bat nears its prey, it emits shorter, lower signals at a faster rate. Order Chiroptera, family Vespertilionidae. |
For reasons not fully understood, all bats are nocturnal, even the fruiteating bats that use vision and olfaction instead of sonar to find their food. The tropics and subtropics have many nectar-feeding bats that are important pollinators for a wide variety of chiropterophilous (“batloving”) plants. Flowers of these plants open at night, are white or light in color, and emit a musky, batlike odor that nectar-feeding bats find attractive.
The famed tropical vampire bat has razor-sharp incisors used to shave away the epidermis of its prey, exposing underlying capillaries. After infusing an anticoagulant to aid blood flow, it laps up and stores its meal in a specially modified stomach.
 |
| Figure 30-20 African lions Panthera leo mating. Lions breed at any season, although predominantly in spring and summer. During the short period a female is receptive, she may mate repeatedly. Three or four cubs are born after gestation of 100 days. Once the mother introduces the cubs into the pride, they are treated with affection by both adult males and females. Cubs go through an 18- to 24-month apprenticeship learning how to hunt and then are frequently driven from the pride to manage themselves. Order Carnivora, family Felidae. |
Reproduction
Reproductive Cycles
Most mammals have definite mating seasons, usually in winter or spring and timed to coincide with the most favorable time of the year for rearing young after birth. Many male mammals are capable of fertile copulation at any time, but female mating function is restricted to a time during a periodic cycle, known as the estrous cycle. Females only copulate with males during a relatively brief period known as heat or estrus. (Figure 30-20).
The estrous cycle is divided into stages marked by characteristic changes in the ovaries, uterus, and vagina. Proestrus, or period of preparation, when new ovarian follicles grow, is followed by estrus, when mating occurs. Almost simultaneously ovarian follicles burst, releasing eggs (ovulation), which are fertilized. In all placental mammals, the fertilized egg implants itself in the uterine wall with pregnancy following. However, should mating and fertilization not occur, estrus is followed by metestrus, a period of repair. This stage is followed by diestrus, during which the uterus becomes small and anemic. The cycle then repeats itself, beginning with proestrus.
How often females are in estrus varies greatly among different mammals. Animals that have only a single estrus during their breeding season are called monestrous; those that have a recurrence of estrus during their breeding season are called polyestrous. Dogs, foxes, and bats belong to the first group; field mice and squirrels are all polyestrous as are many mammals living in the more tropical regions of the earth. Old World monkeys and humans have a somewhat different cycle in which the postovulation period is terminated by menstruation, during which the, endometrium (lining of the uterus) collapses and is discharged with some blood. This menstrual cycle is described in The Reproductive Process.
Reproductive Patterns
There are three different patterns of reproduction in mammals. One pattern is represented by egg-laying (oviparous) mammals, the monotremes. The duck-billed platypus has one breeding season each year. Ovulated eggs, usually two, are fertilized in the oviduct. As they continue down the oviduct, various glands add albumin and then a thin, leathery shell to each egg. When laid, the eggs are about the size of a robin’s egg. The platypus lays its eggs in a burrow nest where they are incubated for about 12 days. After hatching, the young suck milk from the fur of the mother around openings of the mammary glands. Thus in monotremes there is no gestation (period of pregnancy) and the developing embryos draw nutrients stored in their eggs, much as do the embryos of reptiles and birds. But in common with all other mammals, monotremes rear their young on milk.
Marsupials are pouched, viviparous mammals that exhibit a second pattern of reproduction. Although only the eutherians are called “placental mammals,” the marsupials do have a primitive type of placenta, called a choriovitelline (or yolk sac) placenta. The embryo (blastocyst) of a marsupial is at first encapsulated by shell membranes and floats free for several days in the uterine fluid. After “hatching” from the shell membranes, the embryo does not implant, or “take root” in the uterus as it would in eutherians, but it does erode a shallow depression in the uterine wall in which it lies and absorbs nutrient secretions from the mucosa by way of the vascularized yolk sac. Gestation (the intrauterine period of development) is brief in marsupials, and therefore all marsupials give birth to tiny young that are effectively still embryos, both anatomically and physiologically. However, early birth is followed by a prolonged interval of lactation and parental care (Figure 30-21).
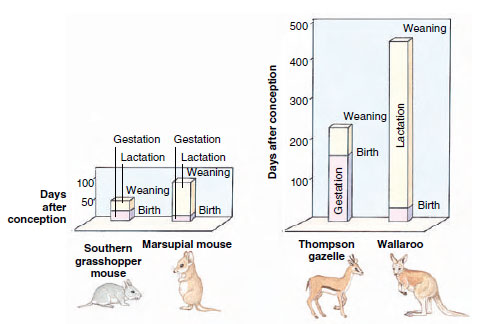 |
| Figure 30-21 Comparison of gestation and lactation periods between matched pairs of ecologically similar species of marsupial and placental mammals. The graph shows marsupials have shorter intervals of gestation and much longer intervals of lactation than in similar species of placentals. |
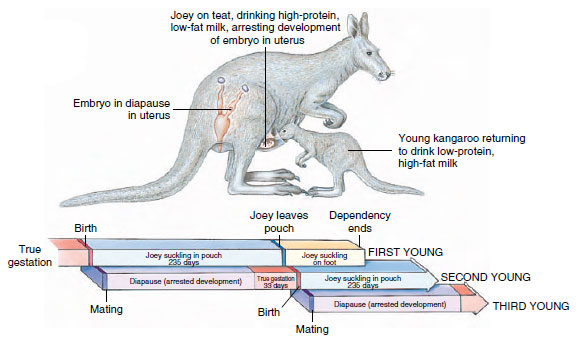 |
| Figure 30-22 Kangaroos have a complicated reproductive pattern in which the mother may have three young in different stages of development dependent on her at once. Order Diprotodontia, family Macropodidae. |
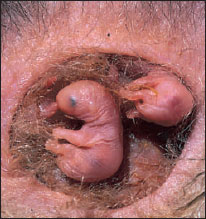 |
| Figure 30-23 Opossums, Didelphis marsupialis, 15 days old, fastened to teats in mother’s pouch. When born after a gestation period of only 12 days, they are the size of honeybees. They remain attached to the nipples for 50 to 60 days. Order Marsupialia, family Didelphidae. |
The third pattern of reproduction is that of viviparous placental mammals, the eutherians. In placentals, the reproductive investment is in prolonged gestation, unlike marsupials in which the reproductive investment is in prolonged lactation (Figure 30-21). The embryo remains in the uterus, nourished by food supplied through a chorioallantoic type of placenta, an intimate connection between mother and young. Length of gestation is longer in placentals than marsupials, and in large mammals it is much longer (Figure 30- 21). For example, mice have a gestation period of 21 days; rabbits and hares, 30 to 36 days; cats and dogs, 60 days; cattle, 280 days; and elephants, 22 months (the longest). But there are important exceptions (nature seldom offers perfect correlations). Baleen whales, the largest mammals, carry their young for only 12 months, while bats, no larger than mice, have gestation periods of 4 to 5 months. The condition of the young at birth also varies. An antelope bears its young well furred, eyes open, and able to run about. Newborn mice, however, are blind, naked, and helpless. We all know how long it takes a human baby to gain its footing. Human growth is in fact slower than that of any other mammal, and this is one of the distinctive attributes that sets us apart from other mammals.
The number of young produced by mammals in a season depends on mortality rate, which, for some mammals such as mice, may be high at all age levels. Usually, the larger the animal, the smaller the number of young in a litter. Small rodents, which serve as prey for many carnivores, usually produce more than one litter of several young each season. Meadow mice are known to produce as many as 17 litters of four to nine young in a year. Most carnivores have but one litter of three to five young per year. Large mammals, such as elephants and horses, give birth to a single young with each pregnancy. An elephant produces, on average, four calves during her reproductive life of perhaps 50 years.
Territory and Home Range
Many mammals have territories—areas from which individuals of the same species are excluded. In fact, many wild mammals, like some humans are basically unfriendly to their own kind, especially so to their own sex during the breeding season. If the mammal dwells in a burrow or den, this area forms the center of its territory. If it has no fixed address, the territory is marked out, usually with the highly developed scent glands. Territories vary greatly in size depending on the size of the animal and its feeding habits. Grizzly bears have territories of several square miles, which they guard zealously against all other grizzlies.
Mammals usually use natural features of their surroundings in staking their claims. These are marked with secretions from the scent glands or by urinating or defecating. When an intruder knowingly enters another’s marked territory, it is immediately placed at a psychological disadvantage. Should a challenge follow, the intruder almost invariably breaks off the encounter in a submissive display characteristic for the species. Territoriality and aggressive and submissive displays are described in more detail in Animal Behavior.
A beaver colony is a family unit, and beavers are among several mammalian species in which the male and female form a strong monogamous bond that lasts a lifetime. Because beavers invest considerable time and energy in constructing a lodge and dam and storing food for winter (Figure 30-24), the family, especially the adult male, vigorously defends its real estate against intruding beavers. Most of the work of building dams and lodges is undertaken by male beavers, but the females assist when not occupied with their young.
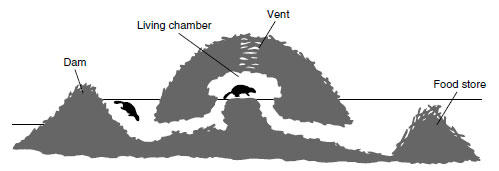 |
Figure 30-24 Each beaver colony constructs its own lodge in a pond created by damming a stream. Each year the mother bears four or five young; when the third litter arrives, the 2-year-olds are driven out of the colony to establish new colonies elsewhere. Order Rodentia, family Castoridae. |
 |
| Figure 30-25 Immature black-tailed prairie dogs, Cynomys ludovicianus, greeting adult. These highly social prairie dwellers are herbivores that serve as an important prey to many animals. They live in elaborate tunnel systems so closely interwoven that they form “towns” of as many as 1000 individuals. Towns are subdivided into family units, each with one or two males, several females, and their litters. Although prairie dogs display ownership of burrows with territorial calls, they are friendly with inhabitants of adjacent burrows. The name “prairie dog” derives from the sharp, doglike bark they make when danger threatens. Order Rodentia, family Sciuridae. |
An interesting exception to the strong territorial nature of most mammals is the prairie dog, which lives in large, friendly communities called prairie dog “towns” (Figure 30-25). When a new litter has been reared, adults relinquish the old home to the young and move to the edge of the community to establish a new home. Such a practice is totally antithetical to the behavior of most mammals, which drive off the young when they are self sufficient.
The home range of a mammal is a much larger foraging area surrounding a defended territory. Home ranges are not defended in the same way as are territories; home ranges may, in fact, overlap, producing a neutral zone used by owners of several territories for seeking food.
Mammalian Populations
A population of animals includes all members of a species that share a particular space and potentially interbreed (Animal Ecology). All mammals (like other organisms) live in ecological communities, each composed of numerous populations of different animal and plant species. Each species is affected by the activities of other species and by other changes, especially climatic, that occur. Thus populations are always changing in size. Populations of small mammals are lowest before the breeding season and greatest just after the addition of new members. Beyond these expected changes in population size, mammalian populations may fluctuate from other causes.
Irregular fluctuations are commonly produced by variations in climate, such as unusually cold, hot, or dry weather, or by natural catastrophes, such as fires, hailstorms, and hurricanes. These are density-independent factors because they affect a population whether it is crowded or dispersed. However, the most spectacular fluctuations are density dependent; that is, they correlate with population crowding.
 |
Figure 30-26 Collared lemming, Dicrostonyx sp., a small rodent of the far north. Populations of lemmings fluctuate widely. Order Rodentia, family uridae. |
Cycles of abundance are common among many rodent species. One of the best-known examples is the mass migrations of the Scandinavian and arctic North American lemmings following population peaks. Lemmings (Figure 30-26) breed all year, although more in summer than in winter. The gestation period is only 21 days; young born at the beginning of the summer are weaned in 14 days and are capable of reproducing by end of summer. At the peak of their population density, having devastated the vegetation by tunneling and grazing, lemmings begin long, mass migrations to find new undamaged habitats for food and space. They swim across streams and small lakes as they go but cannot distinguish these from large lakes, rivers, and the sea, in which they drown. Since lemmings are the main diet of many carnivorous mammals and birds, any change in lemming population density affects all their predators as well.
Varying hares (snowshoe rabbits, Figure 30-7) of North America show 10- year cycles in abundance. The wellknown fecundity of rabbits enables them to produce litters of three or four young as many as five times per year. The density may increase to 4000 hares competing for food in each square mile of northern forest. Predators (owls, minks, foxes, and especially lynxes) also increase (Figure 30-27). Then the population crashes precipitously for reasons that have long been a puzzle to scientists. Rabbits die in great numbers, not from lack of food or from an epidemic disease (as was once believed) but evidently from some density-dependent psychogenic cause. As crowding increases, hares become more aggressive, show signs of fear and defense, and stop breeding. The entire population reveals symptoms of pituitary-adrenal gland exhaustion, an endocrine imbalance called “shock disease,” which results in death. These dramatic crashes are not well understood. Whatever the causes, population crashes that follow superabundance, although harsh, permit the vegetation to recover, providing survivors with a much better chance for successful breeding.
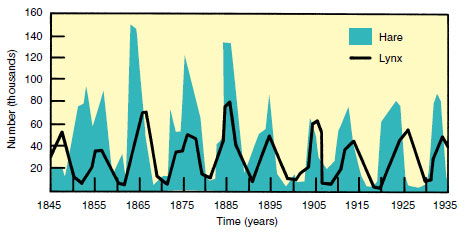 |
| Figure 30-27 Changes in population size of varying hare and lynx in Canada as indicated by pelts received by the Hudson’s Bay Company over a 90-year period. The abundance of lynx (predator) follows that of the hare (prey). |




