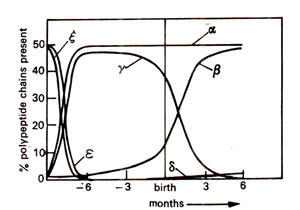
Fig. 44.1. Temporal regulation of the synthesis of different polypeptides representing α and β polypeptide chains of haemoglobin molecules during pregnancy and after birth in human beings.
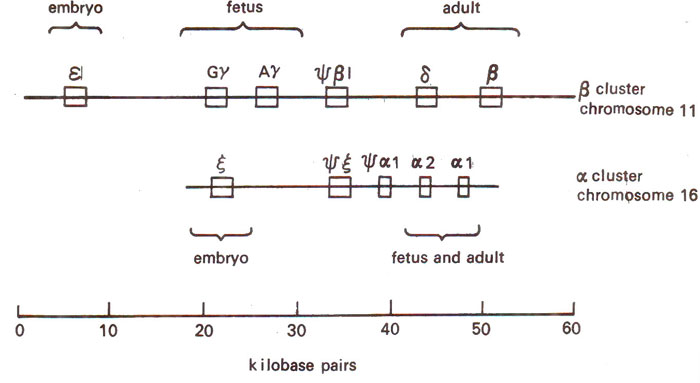
Fig. 44.2. Arrangement of human α and β globin gene clusters on chromosomes 11 and 16.

Fig. 44.3. Phylogenetic relationships among myoglobin and four haemoglobin chains deduced from amino acid substitutions (redrawn from Zuckerkandl, Nature 192, 1961).
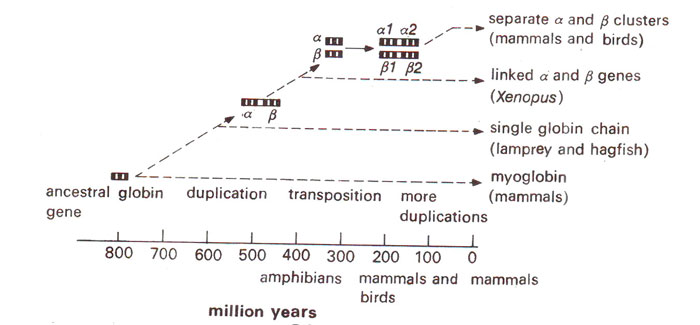
Fig. 44.4. Myoglobin differentiation into α and β chains from earliest period in the history.
If cellular DNA, after digestion, is electrophoresed and hybridized with radioactively labelled rRNA or mRNA product of the gene sequence being analysed or even with genomic or cDNA probe, the number of DNA-RNA or DNA-DNA hybrid molecules formed can be estimated by comparing them with those formed in case of single copy genes. This can be done by measurements of radioactivity in the hybrid molecules by any one of the different methods available. The presence of multiple copies will suggest the presence of a multigene family. Some details of individual multigene families illustrating the above situations will be discussed in the following text.
Multigene families with divergent members Globin gene family in human genome. Although in adult humans only single genes, each specifying α and β polypeptide chains of haemoglobin are expressed, this is not enough during development, such that a whole family of related αglobin genes and another family of β globin genes function. These genes are turned off and on in a coordinated
and sequential manner during embryo development till the fetus is born, depending upon the intracellular milieu. The constitution of haemoglobins found at different stages of development are shown in Table 44.1 and Figure 44.1.
During development, zeta (ζ) is expressed first and is replaced later by a. In beta (β) pathway similarly epsilon (ε) and gamma (γ) are expressed first, which are later replaced by delta (δ) and beta (β). In the adult, α
2β
2 forms 97% of the haemoglobin, α
2 δ
2 forms 2% and α
2 γ
2 (fetal form) forms the remaining 1% due to persistence of the fetal form (Fig. 44.1).

Fig. 44.1. Temporal regulation of the synthesis of different polypeptides representing α and β polypeptide chains of haemoglobin molecules during pregnancy and after birth in human beings.
In humans, a-like globin genes are located on chromosome 16 and β-like globin genes are located on chromosome 11 (Fig. 44.2). Between the genes are
spacer regions (that are not transcribed) and are also present
pseudogenes that are related to globin genes, but are never transcribed. The individual genes of globin gene family are arranged in the order in which they function during the development of fetus (Fig. 44.2), although in other families such an arrangement is not always necessary. It has been shown that in these sequences, the coding regions show little divergence, but spacers in between the genes show considerable diversity as is the case in many other families. The only explanation for this is that the protein function puts a constraint on the evolution of coding sequence, while no such constraint is exercised in spacer region which is fast evolving. Based on the degree of similarity in the amino acid sequences of globin of different mammals, evolutionary trees have also been prepared in the past (Figs. 44.3, 44.4).

Fig. 44.2. Arrangement of human α and β globin gene clusters on chromosomes 11 and 16.

Fig. 44.3. Phylogenetic relationships among myoglobin and four haemoglobin chains deduced from amino acid substitutions (redrawn from Zuckerkandl, Nature 192, 1961).

Fig. 44.4. Myoglobin differentiation into α and β chains from earliest period in the history.
Actin gene family in eukaryotes. Actin genes represent a multigene family, where the members are homologous, but non-identical, giving rise to slightly different variants so that different members function either at different times in the same tissue or in different tissues at the same time. For instance, a minimum of six closely related polypeptides are synthesized in different cells at different times in mammals and the number may be larger in some other systems including flowering plants. The actins may be broadly classified as α
, βand γ actins. Of these, γ actins make contractile apparatus of the muscle, while β and γ actins are constituents of non-muscle cells. There may be variants in each of these three classes, making upto 20-30 actin related sequences in human genome. In a number of plant systems (e.g. tobacco, potato, etc.) also, more than 20 related but variant actin sequences have been reported. In contrast to this, a single actin gene is known in yeast.
Other multigene families with divergent members. Other multigene families having divergent members, that have been studied include genes for the following : (i) albumin and αfetoprotein (major components of blood plasma), serine proteases, the interferons (for defense against viral infection) and the immunoglobulins; (ii) chorion proteins making the major component of egg shell in insects.











