Biochemical mutations in Neurospora
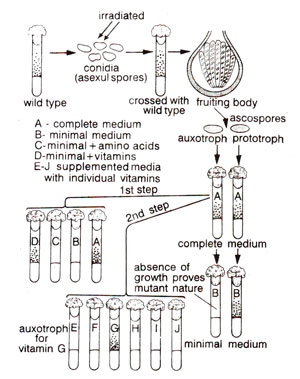
Fig. 22.5. Detection of biochemical mutations in Neurospora.

Fig. 22.6. Replica plating technique for detection and isolation of mutations in microorganisms.
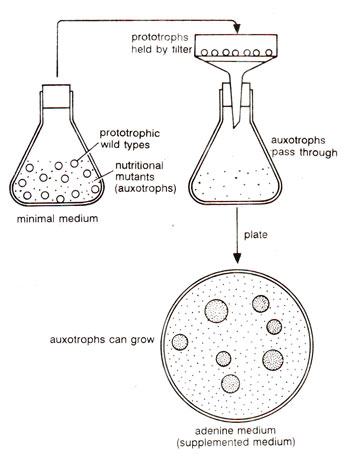
Fig. 22.7. The filtration enrichment method for selecting forwardmutations giving auxotrophs.
Total isolation method
Beadle and Tatum irradiated conidia of Neurospora, mated these irradiated conidia with wild type and subsequently studied segregation for induced mutations, if any. One of the advantages of using Neurospora is that the four products of meiosis stay in same ascus and segregation can be easily studied in eight ascospores of an ascus. For the study of segregation, individual ascospores were first grown on complete medium (a medium having all metabolites in addition to minimal medium), so that both mutant and wild types may grow. These were then transferred to minimal medium. Lack of growth on minimal medium indicated mutant nature. Each mutant culture, thus identified, was then tried on supplemented media (media, containing each specific metabolite in addition to minimal medium) and specific nutritional requirement for which mutation had occurred was thus identified (Fig. 22.5).
This technique originally utilized by Beadle and Tatum, called 'total isolation method' though provides most reliable data, but was very laborious, since individual ascospores had to be tested. Therefore, various modifications were subsequently used by different workers.
One popular modified technique is known as replica plating technique, suggested by J. Lederberg in 1952. This technique can be conveniently utilized not only in Neurospora, but also in other micro-organisms forming colonies. The material (ascospores or spores) is first grown on complete medium. For instance, if streptomycin resistant mutants are to be isolated, material should be allowed to grow on medium lacking streptomycin, so that both mutant and wild types may grow. These colonies are imprinted on velveteen by inverting the petri plate, so that all the colonies are imprinted and leave spores at corresponding positions on velveteen. This will then be the master pattern and other plates having streptomycin can then be pressed on velveteen to get an impression. On this plate now, only mutants for streptomycin resistance will grow. Knowing the position of a mutant colony, corresponding colony in original plate without streptomycin can be marked and used for isolation and multiplication of the mutant. Replica plating technique involving four different steps is illustrated in Figure 22.6.
Concentration of auxotrophs (nutritional mutants) can also be increased by filtration of a culture grown in suspension culture with minimal medium on which only prototrophs will grow. During filtration, due to filamentous growth of fungi, the prototrophs will be filtered out and only non-growing auxotrophic mutant spores will be available in filtrate, which can be subjected to usual method of isolation and testing (Fig. 22.7). The filtration method was devised by Woodward in 1954 and is known as Woodward's filtration technique.
Bacteria and fungi like Neuruspora, in proliferating stage, is sensitive to peniciiiin. Therefore, in a suspension culture with minimal medium, if penicillin is added, the prototrophs (being capable of growing in the minimal medium) will be killed. The auxotrophs will survive due to lack of growth. After killing prototrophs thus, the penicillin can be removed by washing the cells on a filter. If the culture is now plated on a solid medium supplemented with a specific chemical, auxotrophs deficient in this chemical will grow and can be counted and isolated for further study.




