Asparagine Synthetase
(L-Aspartate; Ammonia Ligase E C 6.3.1.4)The Asparagine Synthetase (AS) reaction involves the amidation of aspartate by either glutamine or ammonia.
| 1 Aspartate + glutamine + ATP |
Mg+ + |
asparagine + glutamate + AMP + PPi |
| 2 Aspartate + NH3 + ATP |
Mg+ + |
asparagine 4- H2O + AMP + PPi |
Asparagine synthetase is found in germinating cotyledons and legume plants.
Principle
The enzyme is most easily measured by substituting hydroxylamine for ammonia in the above reaction
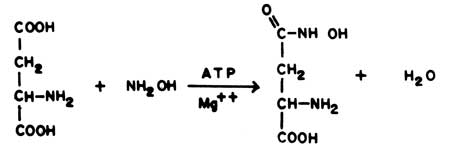 |
Materials
» Tris-NH2OH-MnCl2 SolutionWeigh 2.42g tris base (0.25M), 11.1g hydroxylamine hydrochloride (2M) and 476mg MnCl2.4H2O and dissolve in about 40mL water. Adjust to pH 6.4 by the addition of 8N KOH. Make up the final volume to 80mL. Prepare this reagent fresh.
» Potassium or Sodium ATP, 0.1 M
Dissolve 551mg ATP disodium salt in 10mL water and neutralize it. Prepare fresh solution.
» L-Aspartic Acid, 0.1M, pH 6.4
Dissolve 133mg of L-Aspartic acid in 10mL water and adjust to pH 6.4.
» Ferric Chloride Reagent
Mix 125mL of 20% TCA (20g TCA in 100mL), 50g FeCl3.6H2O and 28mL of Conc. HCl and make up to a final volume of 500mL with water. This is a stable reagent.
» Enzyme Extract
Homogenize 1g chilled plant materials in 10mL 100mM Tris-HCl, pH 8.5 buffer containing 15% (by vol) glycerol, 56mM mercaptoethanol and 4mM KCN. Pass the homogenate through four layers of cheesecloth and centrifuge the filtrate at 15,000g for 30 min.
Procedure
| 1. |
Pipette out 0.4mL of the Tris-NH2OH-MnCl2 solution, 0.1mL of ATP, 0.2mL of enzyme extract. 0.2mL of L-Aspartic acid and 0.1mL distilled water in the order given. |
| 2. |
Incubate at 37°C for 10 min. |
| 3. |
Stop the reaction by the addition of 3mL ferric chloride reagent. |
| 4. |
Centrifuge, and remove the supernatant solution for measurement. |
| 5. |
Measure the absorbance of the supernatant solution at 540nm. |
| 6. |
Use 1mL water and 3mL of ferric chloride reagent for zero adjustment. |
| 7. |
Run a control in which aspartate is omitted from the reaction mixture. |
| 8. |
Prepare a standard curve by taking 0-2.5 micromoles of b-aspartyl hydroxamate. |
Calculation
Express the enzyme activity as micromoles of b-aspartyl hydroxamate formed per mg protein per min. The concentration of b -aspartyl hydroxamate can also be obtained by multiplying the assay absorbance value by a factor of 6.1, if the synthetic one is not available for standardization.References
1. Ravel, J M (1970) In: Methods in Enzymology, XVII, Part A (Ed H Tabor and C W Tabor) Academic Press New York p 722.2. Rogenes, S E (1975) Phytochem 14 1975.




