Indole Acetic Acid Oxidase
Indole acetic acid (IAA) is the best known naturally occurring plant auxin. It participates in controlling many phases of plant growth and differentiation. Levels of free IAA are in turn regulated via synthesis binding, esterification and enzyme degradation. Indolyl acetic acid oxidase (IAAO) is the enzyme involved in the catabolic degradation of IAA to 3-methylene oxindole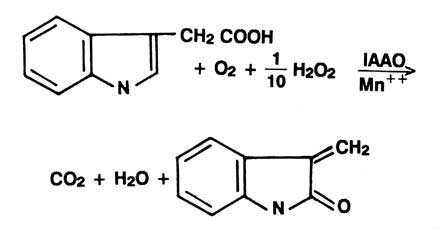 |
Principle
The IAAO activity is determined by measuring residual IAA following dark incubation with shaking at 30°C. The IAA is determined by Salkowski reaction. Since monophenols act as cofactors of IAAO, and o- and p-dihydroxy phenols and polyphenols act as inhibitors of this enzyme, the monophenolic compound, para-coumaric acid is added in the enzyme assay for activation.
Materials
» Phosphate buffer, 0.071M pH 6.2» Para-Coumaric Acid Solution
Dissolve 25mg p-coumaric acid in 50mL water.
» IAA Solution
Dissolve 10mg IAA in 40mL water.
» Manganese Chloride Solution
Dissolve 118mg MnCl2.4H2O in 20mL water.
» Perchloric acid 5M
» Ferric Nitrate, 0.1M
Dissolve 24.18g of substance in 100mL water.
» Enzyme Extract: Prepare acetone powder from the frozen tissue by blender homogenizing 25g tissue in two successive 100mL aliquots of cold acetone. The homogenate was collected by Bűchner filtration through Whatman No. 1 filter paper following both grindings. The homogenate was air-dried until free of acetone odor; the resulting dry powder was weighed and freezer-stored in cold containers.
One g of acetone of powder was ground in two successive 20mL aliquots of 25mM phosphate buffer (pH 6.2) in a mortar chilled in an ice bath. Collect the extract by Bűchner filtration through Whatman No.1 paper after each grinding. Combine the filtrates and dilute to 50mL with phosphate buffer.
Procedure
| 1. |
Pipette out the following solutions in a test tube in the order given below: Phosphate buffer (pH 6.2) 2mL Para-coumaric acid 1mL Manganese chloride 1mL Enzyme extract 2mL |
| 2. |
Start the reaction by the addition of 4mL IAA solution. |
| 3. |
Incubate the reaction mixture in the dark with shaking at 30°C. |
| 4. |
Withdraw 2mL of the mixture after 0 and 50min of incubation and add 5.2mL perchloric acid and 0.5mL ferric nitrate solution. |
| 5. |
Then dilute to 10mL with water. |
| 6. |
After incubating the reaction mixture in the dark for 60min measure the absorbance at 535nm. |
| 7. |
Estimate the protein content in the enzyme extract following the method of Lowry et al. |




