Changing soil pH
Content
Raising soil pH Soil pH can be raised by the addition of lime. Lime is most commonly applied as ground chalk, ground limestone or slaked lime. When lime is added to an acid soil it neutralizes the soluble acids and the calcium cations replace the exchangeable hydrogen on the soil colloid surface (see cation exchange). Eventually hydrogen ions are completely replaced by bases and base saturation is achieved, producing a soil of pH 7 or more. However, care should be taken not to overlime a soil because of its effect on the availability of plant nutrients. The lime requirement of soil can be estimated from knowledge of the required increase in pH and the soil texture (see buffering capacity). A pH of 6.5 is recommended for temperate plants on mineral soils; pH 5.8 on peats. The amount of a liming material needed to meet the lime requirement will depend on the neutralizing value of the lime chosen and its fineness. Liming materials Liming materials can be compared by considering their ability to neutralize soil acidity, fineness, and cost to deliver and spread. The neutralizing value (NV) of a lime indicates its power to overcome acidity. A neutralizing value of 50 signifies that 100 kg of that material has the same effect on soil acidity as 50 kg of calcium oxide. The fineness of the lime is important because it indicates the rate at which it affects the soil acidity (see surface area). It is expressed, where relevant, in terms of the percentage of the sample that will pass through a 100 mesh sieve. Liming materials commonly used in horticulture are listed below with some of their properties. The relationship between the different forms of calcium is shown in Figure 20.5.
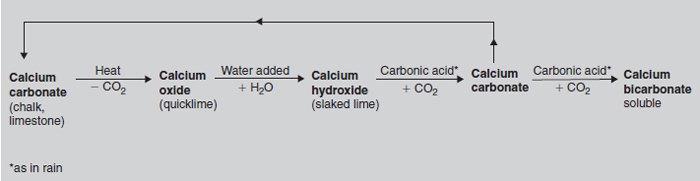
Calcium carbonate is the most common liming material. Natural soft chalk (or limestone) that is high in calcium carbonate is quarried and ground (NV = 48). It is a cheap liming material, easy to store and safe to handle. A sample in which 40 per cent will pass through a 100 mesh sieve can be used at the standard rate to meet the lime requirement. Coarser samples although cheaper to produce, easier to spread and longer lasting in the soil, require heavier dressings. Shell sands, mainly calcium carbonate, have neutralizing values from 25 to 45, i.e. whilst the purest samples can be used at nearly the same rate as chalk, up to twice as much of a poorer sample is required to have the same effect. Calcium oxide (also known as quicklime, burnt lime, cob lime or caustic lime) is produced when chalk or limestone are very strongly heated in a lime kiln. Calcium oxide has a higher calcium content than calcium carbonate and, consequently, a higher neutralizing value. Pure calcium oxide is used as the standard to express neutralizing value (100) and the impure forms have lower values (usually 85–90). If used instead of ground limestone, only half the quantity needs to be applied.
In contact with moisture, lumps of calcium oxide slake, i.e. react spontaneously with water to produce a fine white powder, calcium hydroxide, with release of considerable heat. This was an effective way of obtaining a fine lime from the quarried material before there was heavy rolling machinery to grind the coarse lumps. The lime kilns that were used are still a common sight, especially in small ports round the coast (see Figure 20.6) Although rarely used now, calcium oxide has to be used with care because it is a fire risk, ‘burns’ flesh and scorches plant tissue. Calcium hydroxide, hydrated or slaked lime, is derived from calcium oxide by the addition of water. The fine white powder formed is popular in horticulture. It has a higher neutralizing value than calcium carbonate and its fineness ensures a rapid effect on the growing medium. Once exposed to the atmosphere it reacts with carbon dioxide to form calcium carbonate. It should be noted that all forms of processed lime quickly revert to calcium carbonate when added to the soil. Calcium carbonate, which is insoluble in pure water, gradually dissolves in the weak carbonic acid of the soil solution around the roots (see Figure 20.5). Magnesian limestone, also known as Dolomitic limestone, is especially useful in the preparation of composts because it both neutralizes acidity and introduces magnesium as a nutrient. Magnesium limestone has a slightly higher neutralizing value (50–55) than calcium limestone, but tends to act more slowly. Liming materials also provide the essential nutrients calcium and, when present, magnesium to the soil. Bicarbonate is formed from the carbonate in carbonic acid, e.g. rainwater or soil water, around respiring roots to provide a soluble form that can be taken up by plants (see Figure 20.5). Lime application Unless very coarse grades are used, lime raises the soil pH over a oneto two-year period, although the full effect may take as long as four years; thereafter pH falls again. Consequently lime application should be planned in the planting programme. It is normally worked into the top 15 cm of soil. If deeper incorporation is required, the quantity used should be increased proportionally. The lime should be evenly spread and regular moderate dressings are preferable to large infrequent applications. Very large applications needed in land restoration work should be divided for application over several years. Care should be taken that the surface layers of the soil do not become too acid even when the lower topsoil has sufficient lime. Top layers are the first to become depleted with consequent effect on plant establishment. This tendency has to be carefully looked for in turf management as this can lead to the formation of ‘thatch’ (see Figure 18.6). Applications of organic manures or ammonium fertilizers should be delayed until lime has been incorporated. If mixed they react to release ammonia which can be wasteful and sometimes harmful. Decreasing soil pH Soil pH can be lowered by the addition of acids or sulphur to reduce the base saturation of the mineral soil. Some acid industrial by-products can be used, but the most usual method is to apply agricultural sulphur, which is converted to sulphuric acid by soil micro-organisms. The sulphur requirement depends on the pH change required and the soil’s buffering capacity. The application of large quantities of organic matter gradually makes soils more acid. Acid fertilizers such as ammonium sulphate reduce soil pH over a period of years in outdoor soils and can be used in liquid feeding to offset the tendency of hard water to raise pH levels in composts. In some circumstances it has been appropriate to grow plants in a raised bed of acid peat or to work large quantities of peat into the topsoil; an approach that is not sympathetic to avoiding the destruction of peat wetlands. |
|||||||||||||||||||||||||