Principles of Horticulture / Soil pH
Plant selection
Content
Plant tolerance to soil pH and calcium levels varies considerably, but
all plants are adversely affected when the soil becomes too acid. At very
low pH some elements, such as aluminium, become soluble at levels that
are toxic to plants. Table 20.1 shows the point below which the growth
of common horticultural plants is significantly reduced. Although
aluminium is not an essential nutrient for plants it is required to produce a blue flower rather than a pink one in hydrangeas (see Figure 20.1).
Commercially, growers do not just use a compost with a low pH, but
also add aluminium sulphate to get the blue colouring.
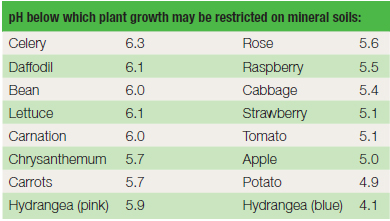 |
| Table 20.1 Soil acidity and plant tolerance |
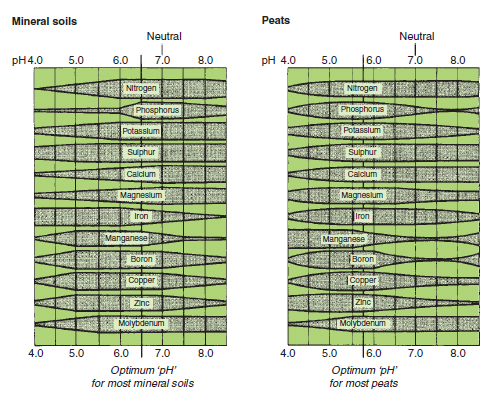 |
Figure 20.4 Effect of soil pH on nutrient availability. The availability of a given amount of
nutrient is indicated by the width of the band. The growing media should be kept at a pH at
which all essential nutrients are available. For most plants the optimum pH is 6.5 in mineral
soils and 5.8 in peats |
In the case of calcifuges the highest point before growth is affected by
the presence of calcium should be noted, e.g. for Rhododendrons and
some Ericas this is at pH 5.5 in mineral soils. Such plants are unable to
metabolize many of the nutrients when there is more calcium present;
typically they show signs of lime induced chlorosis.
In contrast, calcicoles are well adapted to utilizing soil nutrients in the
presence of calcium, but are unable to survive in acid conditions where
they shown signs of aluminium toxicity (dead tissues) and phosphate
deficiency (stunting and blue or reddish stem and leaves).
|
Support our developers







