Developmental genetics
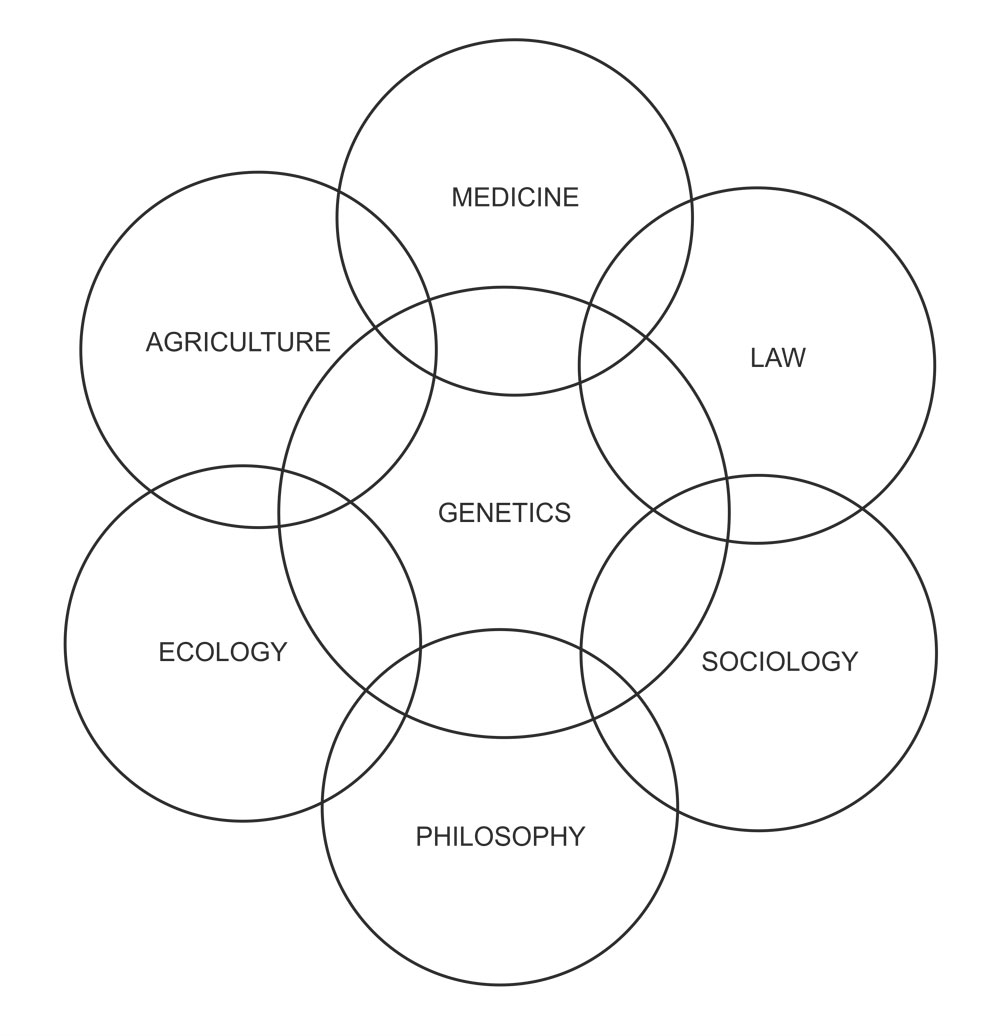
The area of developmental genetics has also received major attention of geneticists in recent years to answer questions like the following. What are the relative roles of nucleus and cytoplasm in differentiation?
Environmental pathogens
This organism is widespread in the environment, but rare in the flora of healthy individuals. Its carriage increases with hospitalization.
Natural Antioxidants in Foods
To protect food quality and safety, antioxidants are often added to processed foods. These antioxidants can be synthetically derived compounds, such as butylated hydroxytoluene and ethylene diaminetetraacetic acid.
Migration of Germ Cells
In vertebrates, the actual tissue from which gonads arise appears in early development as a pair of genital ridges, growing into the coelom from the dorsal coelomic lining on each side of the hind-gut near the anterior end of the kidney (mesonephros).
Algae and Men
Microalgae and macroalgae have been utilized by man for hundreds of years as food, fodder, remedies, and fertilizers. Ancient records show that people collected macroalgae for food as long as 500 B.C. in China and one thousand of years later in Europe.
Macronutrients - Nitrogen
Discovery of the essentiality of nitrogen is often credited to de Saussure (1-3), who in 1804 recognized that nitrogen was a vital constituent of plants, and that nitrogen was obtained mainly from the soil.
Column Chromatography
Column chromatography is one of many forms of chromatography. Others include paper, thin-layer, gas, and HPLC. Most forms of chromatography use a 2-phase system to separate substances on the basis of some physical-chemical property.
Insect Armor
Most insects? tough exoskeletons protect their bodies from predators and from drying out. however, some insects?including young insects, such as caterpillars - have soft bodies. they benefit by adding an extra layer of protective armor.
Agriculture Biotechnology
Biotechnology can be seen as an imprecise term since the harnessing of any biological process could justifiably be called biotechnology.
Distribution of Life on Earth
The biosphere as usually defined is the thin outer layer of the earth capable of supporting life. It is probably best viewed as a global system that includes all life on earth and the physical environments in which living organisms exist and interact.
Animal Defense Mechanism
Packed inside an insect no bigger than a jellybean is a venom strong enough to cause intense pain in humans - and occasionally death, in people who are allergic to it.
Animal Biotechnology
The most important areas of today's research which have potential economic value and prospects of commercialization are the cell and tissue culture based production of vaccines, monoclonal antibodies, pharmaceutical drugs, cancer research, genetic manipul
Prokaryotic DNA polymerases
Three different prokaryotic DNA polymerases are known, of which DNA polymerases I and II are meant for DNA repair and DNA polymerase IN is meant for actual DNA replication
Terrestrial Environments Biomes
A biome is a major biotic unit bearing a characteristic and easily recognized array of plant life. Botanists long ago recognized that the terrestrial environment of the earth could be divided into large units having a distinctive vegetation, such as fores
Types of Mutations
Mutations are heritable changes in the genetic material that give rise to alternative forms of any gene. These alternate forms are called alleles. There are two broad types of mutations, those that affect the gene and those that affect whole chromosomes (
Bioenergetics
An amalgamation of the term biological energetics, is the branch of biology and biochemistry that is concerned with how organisms extract energy from their environment and with how energy is used to fuel the myriad of life?s endergonic processes.




