Distribution of Life on Earth
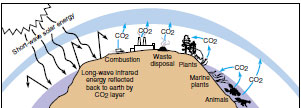 |
| Figure 39-2 “Greenhouse effect.” Carbon dioxide and water vapor in the atmosphere are transparent to sunlight but absorb heat energy reradiated from the earth, leading to warming of atmospheric air |
Biosphere and Its Subdivisions
The biosphere as usually defined is the thin outer layer of the earth capable of supporting life. It is probably best viewed as a global system that includes all life on earth and the physical environments in which living organisms exist and interact. The nonliving subdivisions of the biosphere include the lithosphere, hydrosphere, and atmosphere.
The lithosphere is the rocky material of the earth’s outer shell and is the ultimate source of all mineral elements required by living organisms. The hydrosphere is the water on or near the earth’s surface, and it extends into the lithosphere and the atmosphere. Water is distributed over the earth by a global hydrological cycle of evaporation, precipitation, and runoff. Five-sixths of the evaporation is from the ocean, and more water is evaporated from the ocean than is returned to it by precipitation. Oceanic evaporation therefore provides much of the rainfall that supports life on land. The gaseous component of the biosphere, the atmosphere, extends to some 3500 km above the surface of the earth, but all life is confined to the lowest 8 to 15 km (troposphere). The screening layer in the atmosphere of oxygen-ozone is concentrated mostly between 20 and 25 km. The main gases present in the troposphere are (by volume) nitrogen, 78%; oxygen, 21%; argon, 0.93%; carbon dioxide, 0.03%; and variable amounts of water vapor.
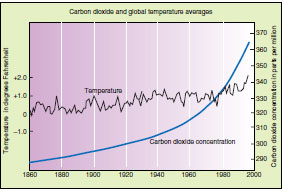 |
| Figure 39-3 Rise in global atmospheric carbon dioxide and global temperature averages for the past 140 years. Data points before 1958 come from analysis of air trapped in bubbles in glacial ice from sites around the world. Atmospheric carbon dioxide has climbed steadily for more than a century while the earth’s temperature has followed a more erratic upward trend. |
Atmospheric oxygen has originated almost entirely from photosynthesis. As discussed in The Origin and Chemistry of Life, the primitive earth contained a reducing atmosphere devoid of oxygen. When oxygen-producing photosynthesis appeared about 3 billion years ago (Figure 39-1), oxygen gradually began to accumulate in the atmosphere. It is believed that by the mid-Paleozoic era, some 400 million years BP, the oxygen concentration had reached its present level of about 21%. Since then, oxygen consumption by animals and plants has approximately equaled oxygen production. The present surplus of free oxygen in the atmosphere resulted from fossilization of plants before they could decay or be consumed by animals. As these vast stores of fossil fuels are burned by our industrialized civilization, the oxygen surplus that accumulated over the ages conceivably could be depleted. Fortunately, depletion is unlikely for two reasons: (1) most of the total fossilized carbon is in the form of noncombustible shales and rocks, and (2) the oxygen reserves in the atmosphere and in the oceans are so enormous that the supply could last thousands of years even if all photosynthetic replenishment suddenly were to cease.
The rapid input of carbon dioxide into the atmosphere from the burning of fossil fuels may significantly affect the earth’s heat budget. Much of the sun’s short-wave light energy absorbed by the earth’s surface reradiates as longer-wave infrared heat energy (Figure 39-2). Materials in the atmosphere, especially carbon dioxide and water vapor, impede this heat loss and allow the atmosphere to warm up. This heating of the atmosphere is called the “greenhouse effect,” since the atmosphere acts to trap reradiated heat from the earth in much the same way the glass of a greenhouse traps heat reradiated by the plants and soil inside. While the greenhouse effect provides conditions essential for all life on earth there is concern that the gradual accumulation of carbon dioxide could lead to an increase in the temperature of the biosphere as a whole (Figure 39-3).




