Mechanism of N2 Fixation and Ammonia Assimilation
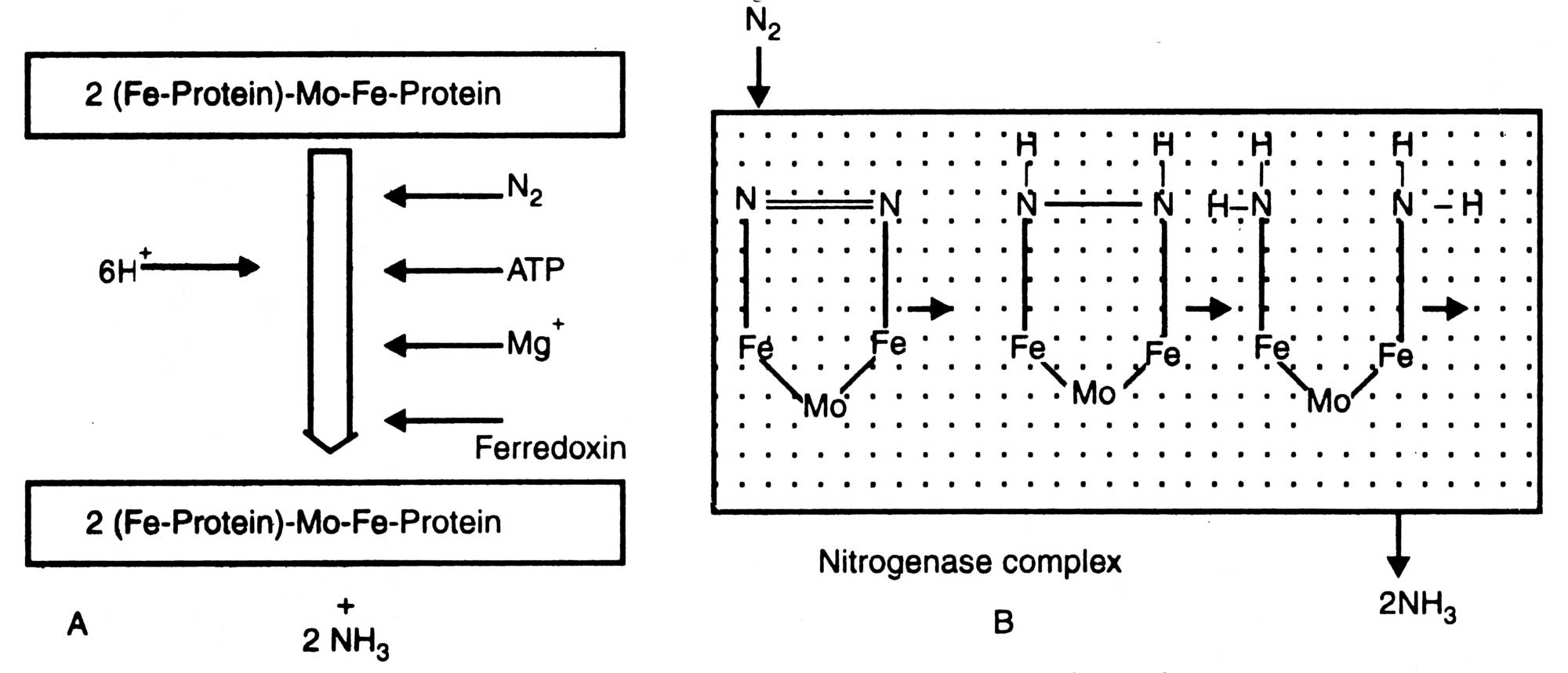
Fig. 11.3. Nitrogen fixation by nitrogenase complex (A) and mechanism of N2 conversion by component I (B).

Fig. 11.4. Mechanism of electron flow during N2 (and other substrate) reduction.
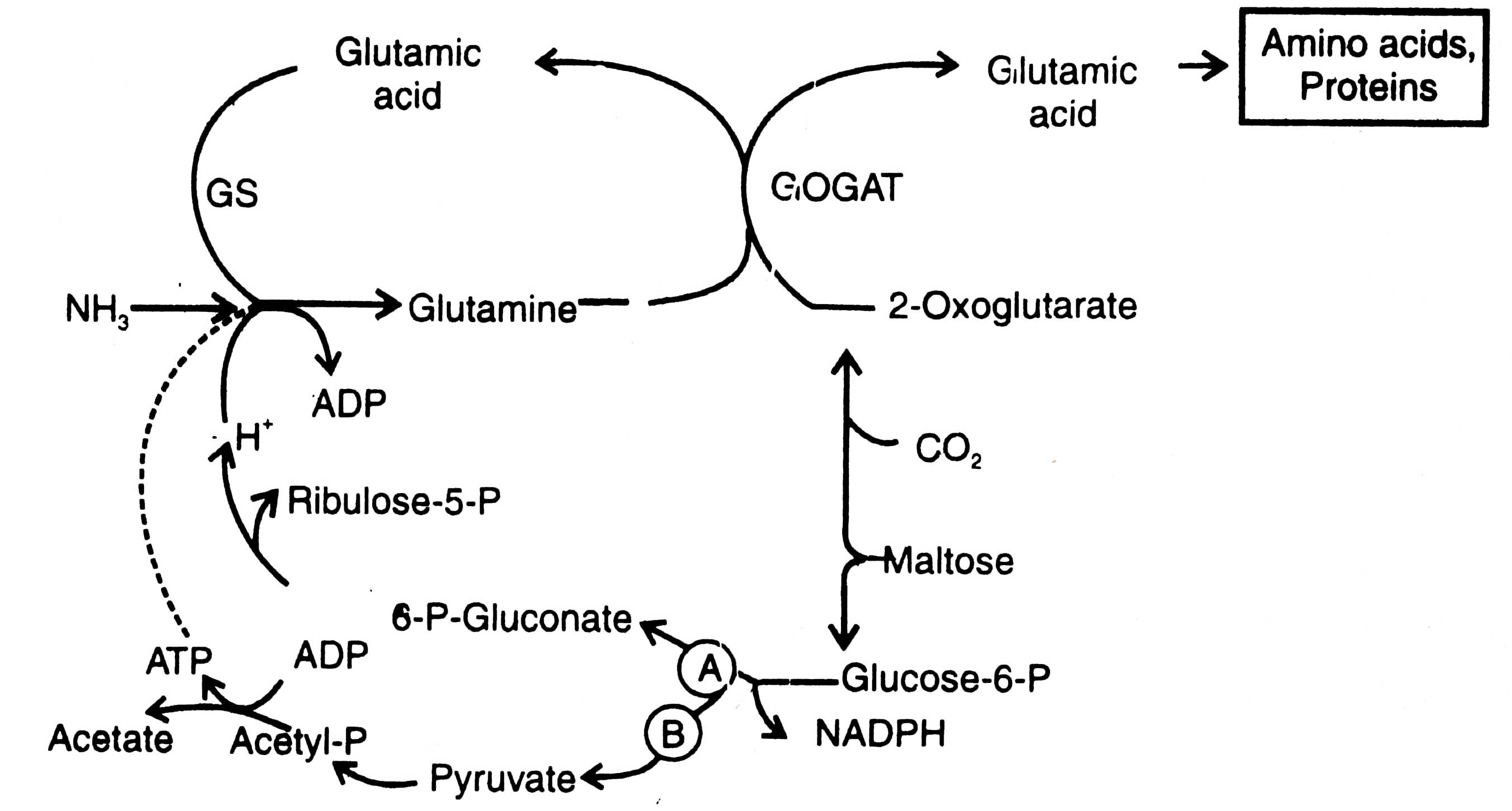
Fig. 11.5. Mechanism of NH3 assimilation in cyanobacteria (A) and bacteria e.g. Clostridium pasteurianum (B). GS-glutamine synthatase; GOGAT-glutamine oxoglutarate aminotransferase.
Mechanism of electron transport is shown in Fig. 11.4. Fe-protein (oxidized form) gets electrons from ferredoxin (when it combines with 2H+ and yields H2) and energy from ATP. Mg++ activates this reaction.
Finally, electron is transferred to oxidize Mo-Fe-protein which becomes reduced and Fe-protein is oxidized. It is the reduced form of Mo-Fe-protein which combines with N2 and other substrates to result in NH3 and other various products with respect to substrate. H2 produced during this reaction is further utilized by some microorganisms which possess hydrogenase. Reutilization of H2 increases nitrogenase activity by protecting it from inhibition of H2.
Ammonia is further synthesized into a number of metabolic products in microbial cells. However, ammonia is not accumulated in the cell, although a few species may create it; rather it is incorporated into organic forms by combining with an organic acid (a -keto-glutaric acid) to give rise to amino acid e.g. glutamic acid. The ammonia may also combine with organic molecules to yield alanin or glutamine (Alexander, 1977).
It is evident from Fig. 11.5 that glutamine synthetase (GS) catalyses the reaction of glutamic acid plus NH3 and converts into glutamine, which in turn combines with 2-oxoglutarate and results in two molecules of glutamic acid in the presence of an enzyme, glutamine oxoglutarate amino transferase (GOGAT). Glutamic acid is the source of several metabolic products such as amino acids, nucleotides, proteins, etc. The 2-oxoglutaric acid is produced by combining maltose with CO2. Also maltose gives rise to glucose-6-phosphate. Its further conversion in different microorganisms differs. In cyanobacteria, glucose-6-phosphate is converted to ribose 5-phosphate with an intermediate product 6-phosphogluconate, and produces H+. In bacteria it differs from genus to genus.
Thus, nitrogen fixed by microorganisms is released into their surrounding. Sewart (1970) proved the release of N15 by Nostoc into sand-dune slack soil.




