Virus structure, classification and antiviral therapy
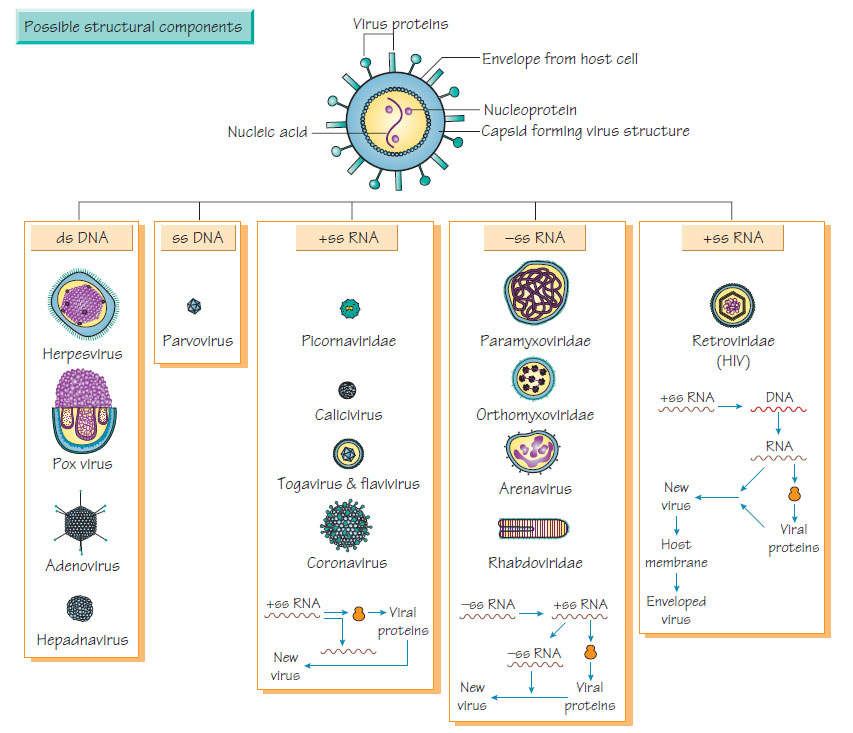
Viral classification is based on the nucleotides in the virus, its mode of replication, the structure and symmetry of the structural proteins (capsids) and the presence or absence of an envelope.Genetic material and replication
DNA viruses
- Double-stranded DNA viruses include poxviruses, herpesviruses, adenoviruses, papovaviruses and polyomaviruses.
- Single-stranded DNA viruses include parvoviruses. DNA viruses usually replicate in the nucleus of host cells by producing a polymerase that reproduces viral DNA. Viral DNA is not usually incorporated into host chromosomal DNA.
RNA viruses possess a single strand of RNA and adopt different reproductive strategies:
- RNA sense (positive) may serve directly as mRNA and be translated into structural protein and an RNA-dependent RNA polymerase.
- RNA antisense (negative) contains an RNA-dependent RNA polymerase that transcribes the viral genome into mRNA. Alternatively, the transcribed RNA can act as a template for further viral (antisense) RNA.
- Retroviruses have single-stranded sense RNA that cannot act as mRNA. This is transcribed into DNA by reverse transcriptase and incorporated into host DNA. The subsequent transcription to make mRNA and viral genomic RNA is under the control of host transcriptase enzymes.
Viral nucleic acid is covered by a protein coat of repeating units (capsids), with either icosahedral (spherical) or helical (arranged around a rotational axis) symmetry. Repeating units reduce the number of genes devoted to production of the viral coat and simplify the process of viral assembly.
Envelope
A lipid envelope derived from host cell or nuclear membrane surrounds some viruses. The host membrane may incorporate viralencoded antigens that may act as receptors for other host cells. Enveloped viruses are sensitive to substances that dissolve the lipid membrane (e.g. ether).
Antiviral therapy
The intracellular location of viruses and their use of host cell systems pose a challenge to the development of antiviral therapy. Drugs may work at different stages of viral replication.
Amantadine/rimantidine prevents uncoating and release of influenza RNA but resistance arises readily. Pleconaril inhibits uncoating of picornaviruses and is active against enteroviruses and rhinoviruses; it is absorbed orally and clinical trials suggest it shortens clinical symptoms.
Nucleoside analogues
Chain termination
Aciclovir is selectively converted into acyclo-guanosine monophosphate (acyclo-GMP) by viral enzymes, then into a potent inhibitor of viral DNA polymerase by host enzymes. The acyclo- GMP causes viral DNA chain termination. Resistance occurs through the development of deficient thymidine kinase production or alteration in the viral polymerase gene. The drug can be taken orally and crosses the blood-brain barrier. Other agents (e.g. ganciclovir) work in a similar way.
Lamivudine inhibits the reverse transcriptase of hepatitis B and HIV (see below). Nucleoside and nucleotide inhibitors are being developed as alternative treatments for hepatitis B; these include adefovir, entecavir, tenofovir, telbivudine and clevudine. Ribavirin is a guanosine analogue that inhibits several steps in viral replication including capping and elongation of viral mRNA. It is active against respiratory syncytial virus, influenza A and B, parainfluenza virus, Lassa fever, hantavirus and other arenaviruses.
Nucleoside reverse transcriptase inhibitors
Nucleoside reverse transcriptase inhibitors (NRTIs) inhibit reverse transcriptase by being incorporated as faulty nucleotides. Examples include the longest established antiretroviral drug zidovudine (AZT), plus lamivudine (3TC), stavudine (d4T), tenofovir, didanosine (ddI) zalcitabine (ddC) and abacavir (see HIV infection and AIDS).
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) inhibit reverse transcriptase directly; examples include nevirapine, efavirenz, delavirdine and etravirine. They have been shown to be effective agents in combination regimens. As resistance occurs after a single mutation, they are used in maximally suppressive regimens only.
Protease inhibitors
Protease inhibitors target the HIV-encoded protease. They are highly effective antiretroviral compounds that cause significant falls in viral load. They include atazanavir, indinavir, lopinavir, ritonavir and saquinavir. Ruprintrivir acts in the same way against human rhinovirus 3C protease. It is administered by nasal spray and appears to have useful activity in rhinovirus infection.
Fusion inhibitors
Enfuvirtide inhibits binding with gp134; maraviroc inhibits binding to CCR5 preventing fusion. Both agents are used for salvage therapy in AIDS (see HIV infection and AIDS).
Neuraminidase inhibitors including zanamivir and oseltamivir inhibit the final stage in the release of virus from the host cell.
Integrase inhibitors
These agents are being developed to block the insertion of the HIV viral genome into the DNA of the host cell.
Other agents
Infections with hepatitis B and hepatitis C can also be treated with α-interferon, a host cytokine.