The Polymerase Chain Reaction (PCR)
Amplification of DNA
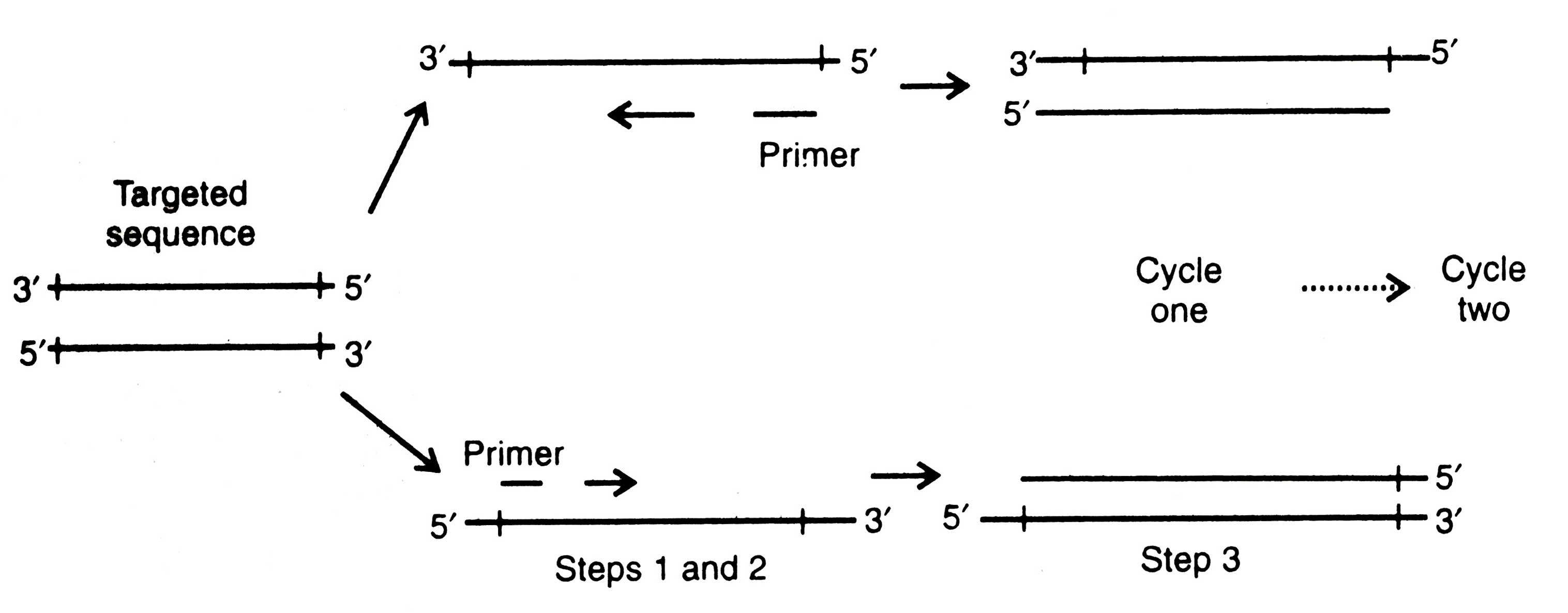
The PCR includes the following three essential steps to amplify a specific DNA sequence (Fig. 2.15).
(i) Melting of target DNA. The target DNA containing sequence (between 100 and 5,000 base) to be amplified is heat denatured (around 94°C for 15 second) to separate its complementary strands (step 1). This process is called melting of target DNA.
(ii) Annealing of primers. The second step is the annealing of two oligonucleotide primers to the denatured DNA strands. Primers are added in excess and the temperature lowered to about 68°C for 60 seconds consequently the primers form hydrogen bonds i.e. anneal to the DNA on both sides of the DNA sequence (step 2).
However, after completion of step 3 (of one cycle) the targeted sequences on both strands are copied and four strands are produced. Now, the three step cycle (first cycle) is repeated which yields 8 copies from four strands.
The PCR technology has been improved in recent years. RNA can also be efficiently used in PCR technology. The rTth DNA polymerase is used instead of the Taq polymerase. The rTth polymerase will transcribe RNA to DNA, thereafter amplify the DNA. Therefore, cellular UNA and RNA viruses may be studied when they are present in small quantities.
Application of PCR Technology
The PCR technology is extensively applied in the following areas (of molecular biology, medicines and biotechnology):
| (a) | Amplification of DNA and RNA |
| (b) | Determination of orientation and location of restriction fragments relative to one another. |
| (c) | Diagnosis of diseases and causal microorganisms. For example, PCR-based diagnostic tests for AIDS, Chlamydia, tuberculosis, hepatitis, human papilloma virus, and other infectious agents and diseases are being developed. The tests are rapid, sensitive and specific. |
| (d) | The PCR is important in detection of genetic diseases such as sickle cell anaemia, phenylketonuria and muscular dystrophy. |
| (e) | It is most applicable in forensic science where it is being used in search of criminals through DNA fingerprinting technology. However, the feasibility of fingerprinting is now being challenged in court of law. In these cases only small samples of biological materials are required. |
| (f) | It is also applied in diagnosis of plant diseases. A large number of plant pathogens in various hosts or environmental samples are detected by using PCR, for example, viroids (associated with hops, apple, pear, grape, citrus, etc), viruses (such as TMV, cauliflower mosaic virus, bean yellow mosaic-virus, plum pox virus, polyviruses), mycoplasmas, bacteria (Agrobacterium tumifaciens, Pseudomonas solanacearum, Rhizobium leguminosarum, Xanthomonas compestris, etc), fungi (e.g. Colletotrichum gloeosporioides, Glomus spp; Laccaria spp., Phytophthora spp, Verticillium spp), and nematodes (e.g. Meloidogyne incoginta, M. javanica, etc) (Henson and French, 1993; Chawla, 1998). |
| (g) | PCR is finding considerable and unique use in archaeology; it is doubtful whether scientists will be able to resurrect wooly mammoth and dinosaurs from the remains of ancient animals as recently epitomized in Michael Crighton's Jurassic Park. |