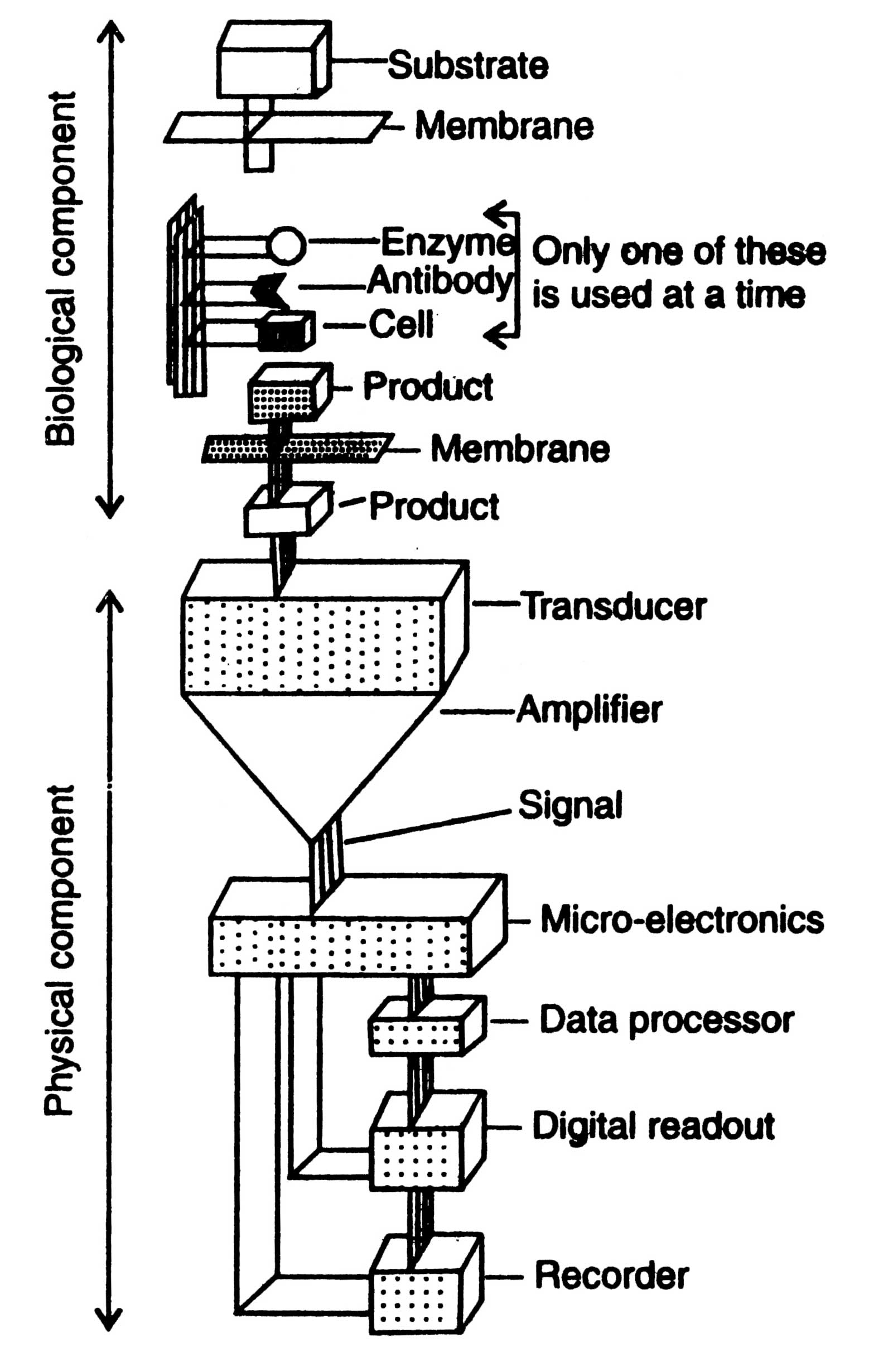
Biosensor
The principle of the biosensor is quite simple (Fig. 17.6). The biological material is immobilized as described earlier on the immobilization support, the permeable membrane, in the direct vicinity of a sensor. The substances to be measured pass through the membrane and interact with the immobilized material and yield the product. A product (i.e. the monitored substrate) may be heat, gas (oxygen), electrons, hydrogen ions or the product of ammonium ions. The product passes through another membrane to the transducer. The transducer converts product into an electric signal which is amplified. The signal processing equipment converts the amplified signals into a display most commonly the electric signal which can be read out and recorded.
A glucose-electrode is shown in Fig. 17.7. It can be built up by immobilizing glucose oxidase in polyacrylamide gel around a platinum oxygen-electrode separated by a teflon membrane. KCl solution is placed around the platinum-oxygen electrode. From upper surface glucose oxidase is intimately covered by a cellulose acetate membrane.
When glucose solution is brought into the contact of membrane, glucose and oxygen pass through membrane into the enzyme layer and, as a result of oxidation-reduction reactions, converted into gluconic acid and hydrogen peroxide in the presence of water, oxygen and glucose oxidase. Consequently, oxygen concentration in the gel around the electrode is lowered down. Hydrogen peroxide brings about a change in current i.e. measurable signal. Electrode records the rate of reactions. The rate of diminition of oxygen concentration is proportional to glucose concentration of the sample. It responds linearly to glucose concentrations over a range of 10-1-10-5mol dm-3 with a response time of 1 minute.
In 1987, for the first time Yellow Springs Instruments Co., USA developed a biosensor for diagnostic purposes for measuring glucose in blood plasma. It is a hand - held machine which measures six components of blood plasma for example, glucose, urea, nitrogen, sodium, potassium and chloride.
An indigenous glucose sensor has been developed by the scientists at Central Electrochemical Research Institute (CECRI), Karaikudi. It gives electrical signal for a glucose concentration as low as 0-15 millimoles.
Biosensor are of different types based on the use of different biological material and sensor devices; a few of them are discussed below:
(i) Electro-chemical biosensor. This type of biosensor has been developed by using electronic devices such as field effect transmitors or light emitting diode; the former measures charge accumulation on their surface and the later photoresponse generated in a silica based chip as an alternating current. Hence, the field effect transmitor measures a biochemical reaction at the surface and induce into current (Gronow et al, 1988). Moreover, the field effect transmitors can be modified to ion sensitive, enzyme sensitive or antibody sensitive ones by using selective ions, enzymes or antibodies respectively.
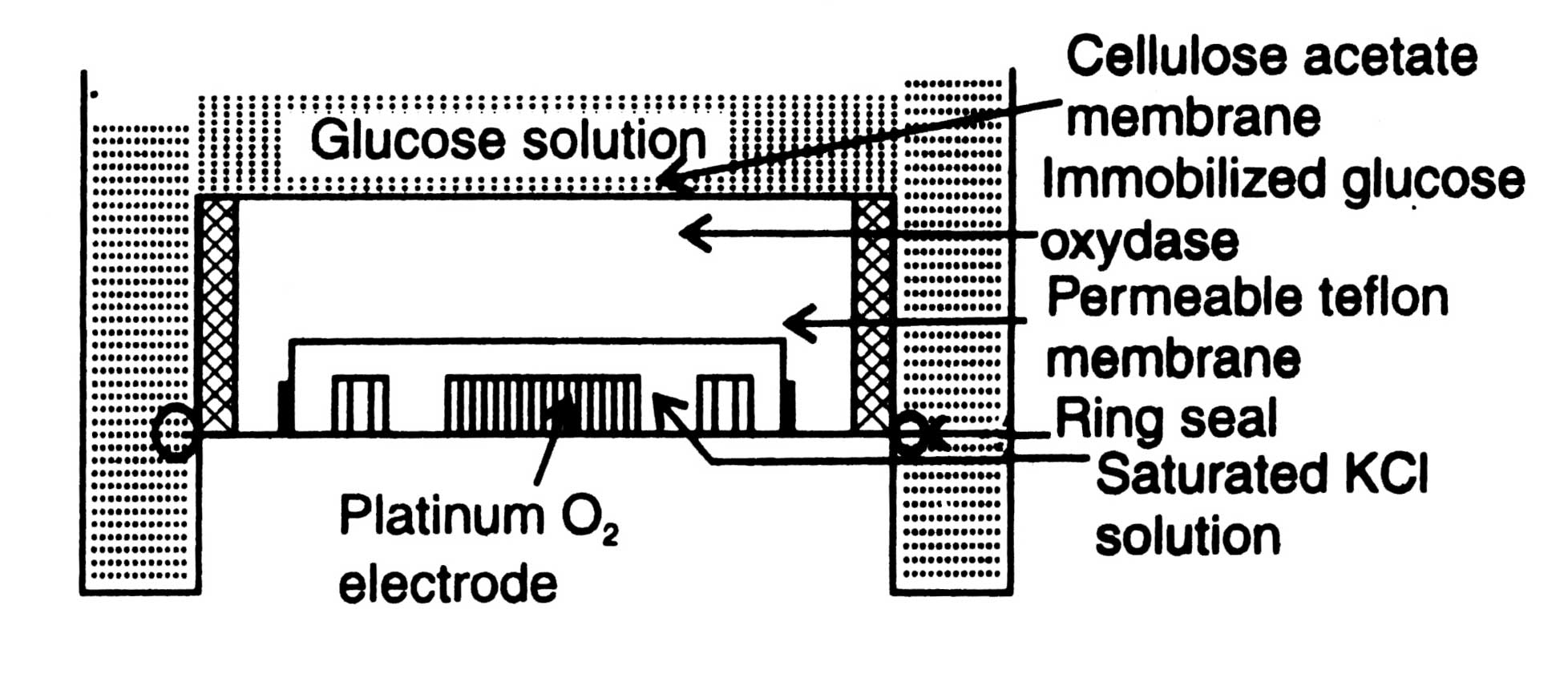
Fig. 17.7. A glucose electrode
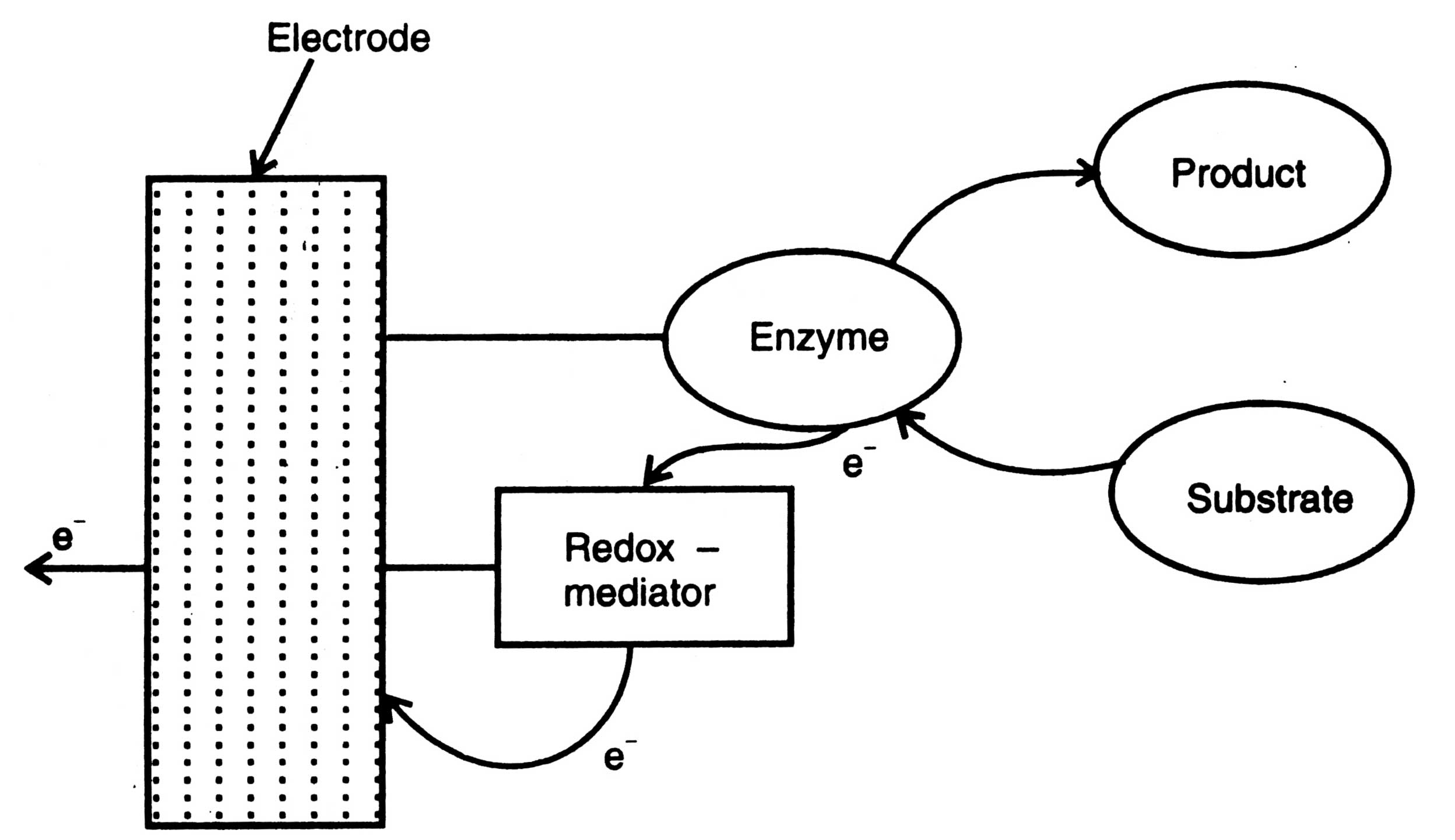
Fig. 17.8. Mediated biosensor.
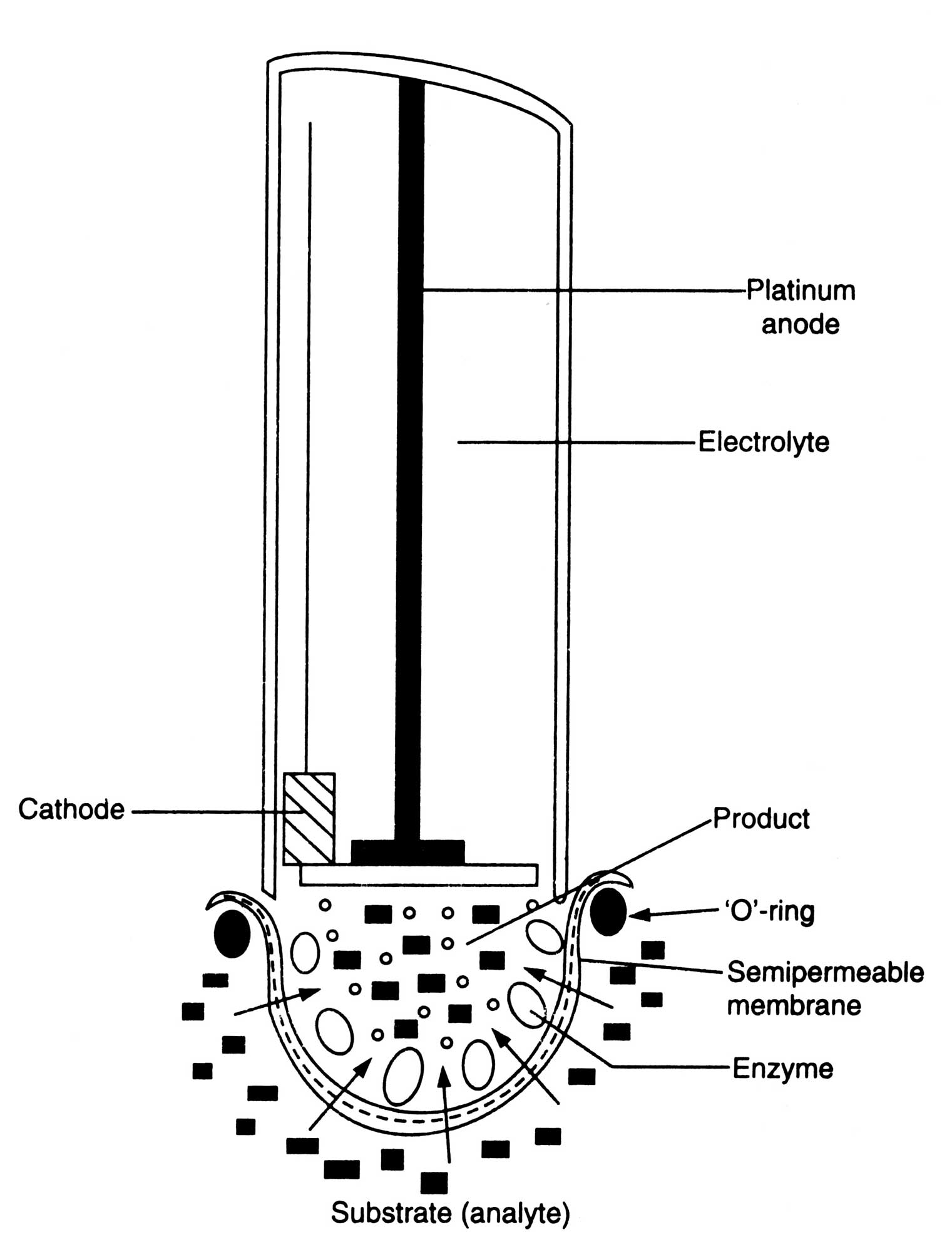
Fig. 17.9. A simple biosensor combining on electrochemical electrode and an enzyme immobilized on to a semipermeable membrane.
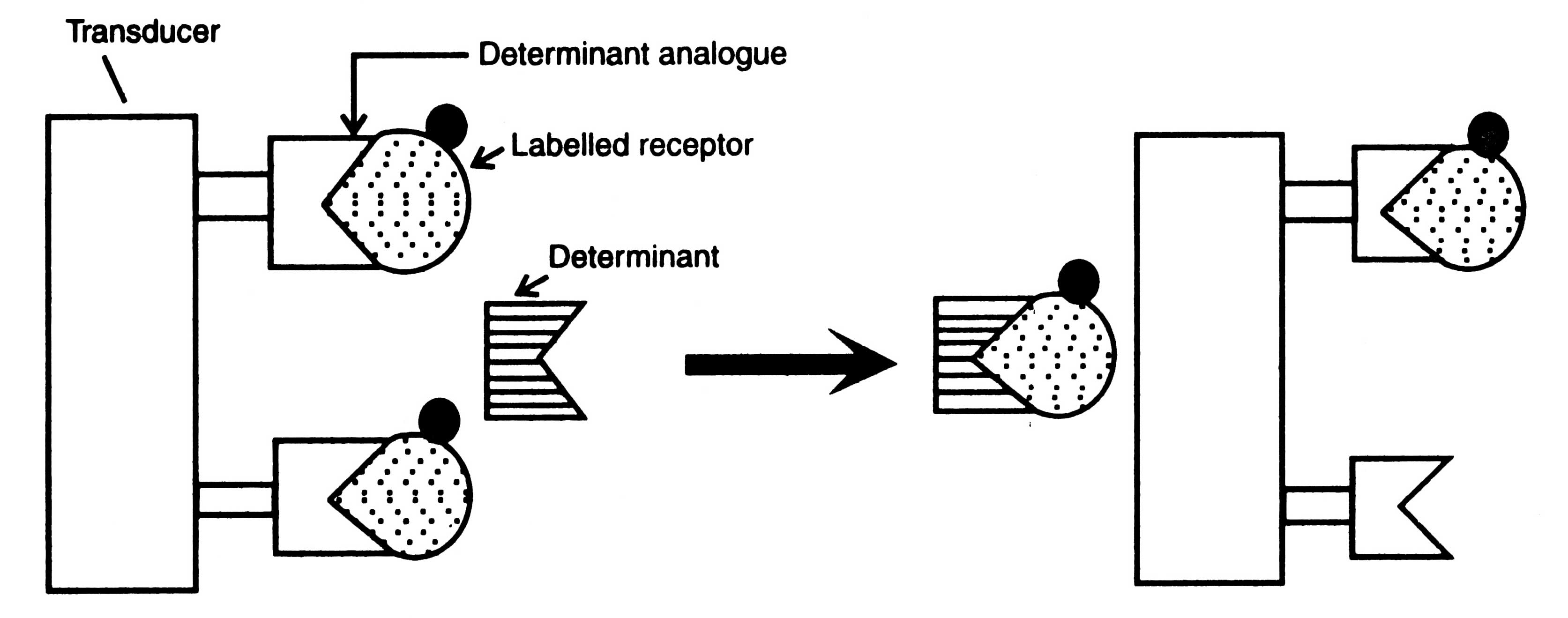
Fig. 17.10. Principle of bioaffinity sensor.
(ii) Amperometric biosensor. Amperometric biosensors are those which measure the reaction of anylate with enzyme and generate electrons directly or through a mediator. The amperometric biosensors contain either enzyme-electrode or without a mediator, or chemically modified electrodes. The oxygen and peroxide based biosensor and others (Table 17.4) discussed earlier are enzyme -electrode biosensor. Some advancement has been brought into this type of biosensor by using a mediator. In addition, more advanced types are the direct electron transfer systems. Principle of a mediated biosensor is shown in Fig. 17.8.
In this biosensor, a redox reaction catalyzed by an enzymes is directly coupled to an electrode where enzyme is presented with the oxidizable substrate. The electrons are transferred from the substrate to the electrode via enzyme and redox mediator. In this biosensor the oxidase replaces the oxygen requirement of the enzymes.
Enzyme electrodes. Enzyme electrodes are a new type of biosensors which have been designed for the amperometric assay of potentiometric assay of substrates such as urea, amino acid, glucose, alcohol, and lactic acid. The electrode consists of a given electrochemical sensor in close contact with a thin permeable enzyme membrane capable of reacting with the given substrates. The enzyme is embedded in the membrane and produce O2, H+, NH4+, CO2 or other small molecules depending on enzymatic reactions. This is detected by the specific sensor. The magnitude of the response determines the concentration of substrates (Fig. 17.9).
(iii)Thermistor containing biosensor. Thermistor is used to record even a small temperature changes (between 0.1-0.001°C) during biochemical reactions. By immobilizing enzymes like cholesterol oxidase, glucose oxidase, invertase, tyrosinase, etc. thermistors have been developed (Gronow et al, 1988). Moreover, thermistors are also employed for the study of antigen- antibody with very high sensitivity (10-13 mol dm-3) in case of thermometric Enzyme Linked Immunoabsorbant Assay (ELISA).
In this biosensor, a receptor is radiolabelled and allowed to bind with determinant analogue immobilized onto the surface of a transducer. When concentrations of a determinant are increased, the labeled receptor forms an intimately bound complex with determinant.
Table 17.3. Typical enzymes based biosensors.
| Substance | Enzymes | Response time |
Range |
| Amines (for meat freshness) |
Monoamine oxidase | 4 min | 50-200 m mol dm-3 |
| Cholesterol | Cholesterol oxidase | 2 min | 10-2 - 3x10-5 mol dm-3 |
| Carbon monoxide | CO : acceptor | 15 sec | 0-65 m mol dm-3 |
| Glucose | Glucose oxidase | 20 sec | 2 x 10-3 -3x10-6 mol dm-3 |
| Penicillin | Penicillinases | 25 sec | 1-10 m mol dm-3 |
| Sucrose | Invertase | 6 min | 10-2 -2x10-3 mol dm-3 |
| Uric acid | Uricase | 30 min | 5x10-3-5x10-5 mol dm-3 |
(v) Whole cell biosensors — (Microbial Biosensors). In this device, either immobilized whole cell of microorganisms or their organelles are used. These react with a large number of substrates and show generally slow response (Corcoran and Rechnitz, 1985). Immobilized Azotobacter vinelandii coupled with ammonia electrode shows sensitivity range between 10-5 and 8 x 10 mol dm-3. It measures the concentration of nitrate within 5-10 min-2. Examples of microbial biosensors are given in Table 17.4.
Table 17.4. Microbial biosensors containing oxygen electrode.
| Microorganism | Sensoring for | Response time(min) |
Range |
| Brevibacterium lactofermentum |
Assimilase sugars | 1-10 | Linear above 1 m mole dm-3 |
| Bacillus subtilis | Mutagen screening | 90-100 | 1-6m g cm-3 |
| Methylomonas flagellata |
Methane | 1 | upto 6.6m mol dm-3 |
| Trichosporon brassicae |
Acetate | 6-10 | Linear upto 22.5 mg dm-3 |
| T. brassicae | Ethanol | 10 | below 22.5 mg dm-3 |
Applications of biosensor
In the beginning biosensor was applied in the field of medicine and industry. But in recent years, biosensors are becoming popular in many areas due to the small size, rapid and easy handling, low cost, and greater sensitivity and selectivity. Application of biosensor in some of the areas is described as below:
(ii) Uses in pollution control. Biosensors are very helpful in environmental minitoring and pollution control, since they can be miniaturized and automated. As far as quality control of drinking water is concerned, the minitoring biosensors are successful in monitoring of pesticides in water. In Japan, a biosensor coupled with oxygen electrode and immobilized Trichosporon cutaneum is used for measuring biological oxygen demand (BOD) (Gronow et al, 1988).
The whole cell biosensor developed by immobilizing Salmonella typhimurium and Bacillus subtilis in conjugation with oxygen electrode can be used to measure mutagenicity and carcinogenecity of several chemical compounds.