Enzyme Technology
Applications of Enzymes
Therapeutic Uses
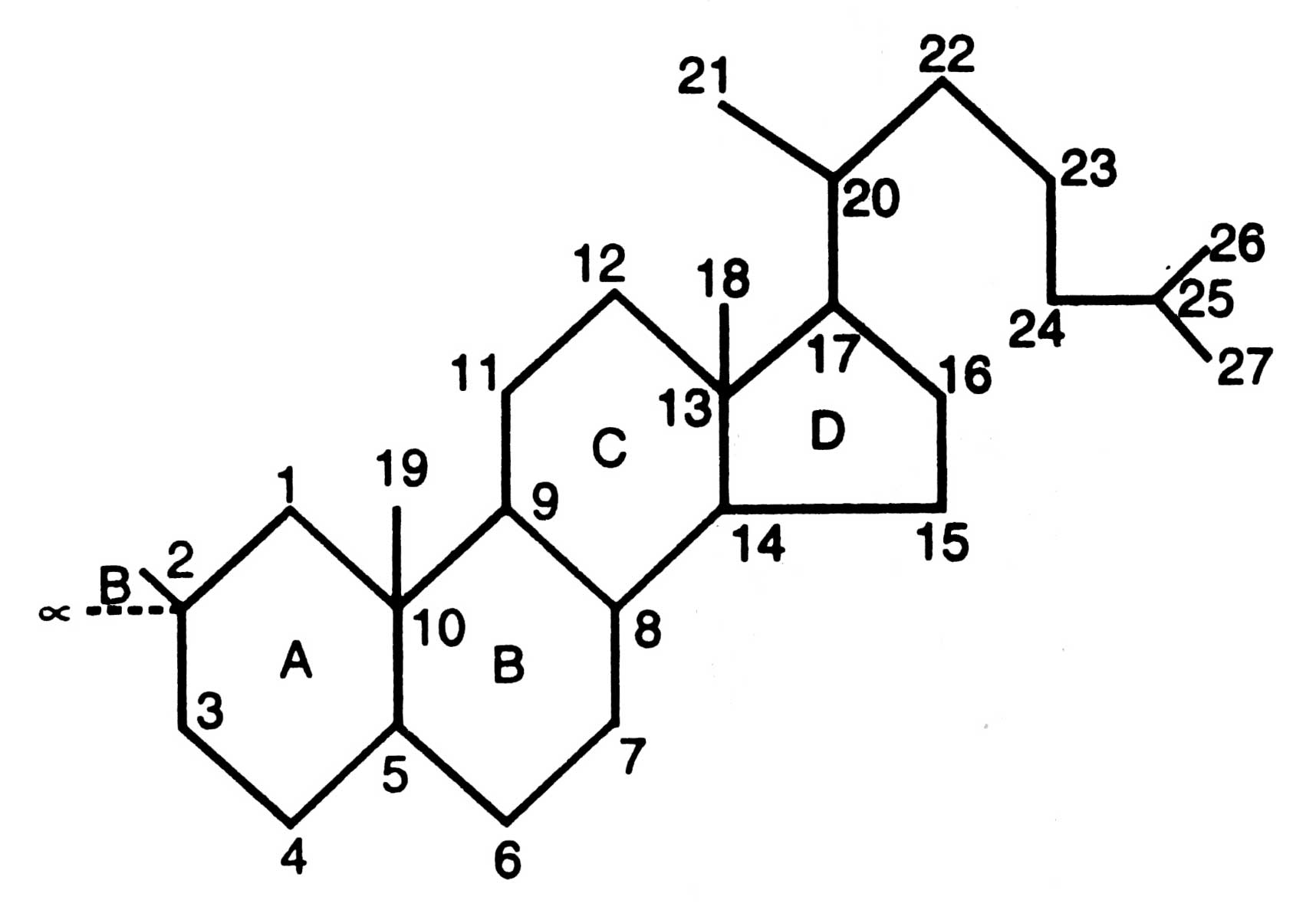
Enzymes are used for this purpose where some inborn errors of metabolism occur due to missing of enzyme (see Monoclonal antibodies) where specific genes are introduced to encode specific missing enzymes. However, in most of cases certain diseases are treated by administering the appropriate enzyme. For example, virilization of a disease developed due to loss of an hydroxylase enzyme from adrenal cortex and introduction of hydroxyl group (-OH) on 21-carbon of ring structure of steroid hormone. Steroids are compounds having a common skeleton in the form of perhydro-1, 2-cyclo-pentano-phenanthrene (Fig. 17.4). The missing enzyme synthesizes aldosterone (male hormone) in excess leading to masculinization of female baby and precocious sexual activity in males in about 5-7 years.
Analytical Uses
Use of enzymes for analytical purposes is also important. Generally end point and kinetic analysis are possible. End point analysis refers to total conversion of substrates into products in the presence of enzymes in a few minutes while kinetic analysis involves the rate of reaction and substrate/product concentration. Moreover, analysis of antibodies, immunoglobins, necessary for human use poses a great promise. The usable enzymes are alkaline phosphatase, b-galactosidase, b-lactamase, etc.
Another use of enzyme is in biosensor, device of biologically active material displaying characteristic specificity with chemical or electronic sensor to convert a biological compound into an electronic signal. It is constructed to measure almost anything from blood glucose. A simple carbon electrode, an ion sensitive electrode, oxygen electrode or a photocell, may be a sensor (Trevan, 1987).
Manipulative Uses
A variety of enzymes isolated from different sources are now-a-days applied in genetic engineering as one of the biological tools. Some of them are available in market (Table 3.1). Examples and brief discussion of these enzymes are given in Example of some enzymes.
Enzymes are used in industries in different ways.
In dairy industry
For a long time calf rennet has been used in dairy industry. In recent years, calf rennets are replaced by microbial rennets (e.g. Mucor michei). They are acid aspartate proteases. They slightly differ from calf rennets as they depend for reaction with casein on Ca++, temperature, pH, etc.
Lactase (produced by Bacillus stearothermophilus) is used for hydrolysis of lactose in whey or milk, and lipase for flavor development in special cheeses.
In detergent industry
During normal washing proteinaceous dirt often precipitates on solid cloths and proteins facilitate to adhere the dirt on textile fibers and make stains on cloths. These stains are difficult to remove from clothes. Nevertheless, it can be easily removed by adding proteolytic enzymes to the detergent. It attacks on peptide bonds and therefore, dissolves protein. The alkaline serine protease obtained from B. licheniformis is most widely preferred to use in detergent. In addition, the serine protease of B. amyloliquefaciens is also used for this purpose. It contains a-amylase, hence to some extent it may be advantageous (Aunstrup et al., 1979).
It has been mentioned earlier that hydrolysis of starch began in early 1960s to prepare dextrose and glucose syrups. Furthermore, for complete acid hydrolysis of starch to dextrose glucoamylase was coupled with bacterial a-amylase. Currently, various enzymatic processes are applied for various products (Fig. 17.5).
Glucose isomerase is an important enzyme used commercially in conversion of glucose to fructose via isomerization. Fructose is used in the preparation of fructose syrup.
The reaction mixture at the end contains 42% fructose, 52% glucose and 6% dextrins. The mixture is sweeter than glucose and as sweet as sucrose. Now, techniques have been developed to obtain 55% fructose concentration in syrup (Singh, 1987).
In brewing industry
Enzymes used in brewing industry are a-amylase, b-glucanase and protease which are required for malt in substitution of barley. Source of these enzymes is B. amyloliquefaciens. a-amylase is not required for liquefaction or brewing adjuncts and b-glucanase alleviates filtration problems due to poor malt quality and neutral protease helps in the inhibition of alkaline protease by an inhibitor.
In wine industry
Pectic enzymes are used in wine industry for high yield of products of improved quality. The pectic enzymes are pectin transeliminase (PTE), polymethyl galacturonase (PMG), polygalacturonase (PG),pectine esterase (PE), etc. However, pectic enzymes give a good result when combined with other enzymes e.g. protease glucoamylase, etc.
In pharmaceutical industry
Penicillin G/V acylase, glucose isomerase, etc. are widely used in Pharmaceuticals for the production of semisynthetic penicillins and fructose syrup, respectively. All penicillins consist of an active beta lactam ring i.e. 6-amine penillanic acid (6 APA) group combined with different side chains (R group) (Fig. 16.2) Penicillin G/V acylase removes G/V group from penicillin G/V resulting in separation of 6 APA and R groups.
Finally, new synthetic side chains are coupled with 6 APA to synthesize new semisynthetic penicillins. Enzyme reaction are as below:
| penicillin G acylase | ||
| Penicillin G |  |
6APA + G side chain |
| penicillin V acylase | ||
| Penicillin V |  |
6APA + V side chain |
| side chain addition | ||
| 6APA |  |
semisynthetic penicillins |