Qualitative Analysis
The identification of the 13 unknowns is based on a flow chart that can be followed as presented here to separate and identify the unknowns. NaCl, NaHCO3, Na2CO3, NaC2H3O2, NH4Cl, MgSO4, Ca(NO3)2, H3BO3, CaCO3, CaSO4, sucrose, cornstarch, and fructose.
Add Water to Each
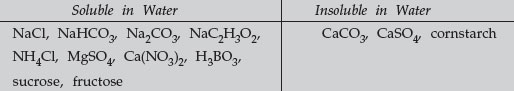
Test for Insoluble Substances
- CaCO3 bubbles with vinegar - produces CO2 gas.
- Cornstarch turns purple with iodine, the other 2 are brownish-colored.
- CaSO4 – the one lef
Test for Soluble Substances
- NH4Cl - acidic pH in solution, solid in a container will produce a slight ammonia smell when opened sometimes.
- Na2CO3, NaC2H3O2—both produce pink solutions with phenolphthalein but only Na2CO3 bubbles with vinegar, so test the 2 pink solutions with vinegar; one bubbles and the other does not.
- NaHCO3 bubbles with vinegar but does not produce pink solution with phenolphthalein; should produce slightly basic pH in water but not much.
- MgSO4, Ca(NO3)2 - MgSO4 produces distinct precipitate with NaOH solution, Ca(NO3)2 produces milky appearance in solution with NaOH - essentially lime water - but not a distinct precipitate like MgSO4.
- Fructose produces a red precipitate with Benedict’s test or copper(II) sulfate.
- At this point NaCl, sucrose, and H3BO3 is left, which is very soluble in alcohol, but the other 2 are not. Sucrose dissolves readily in warm water, while NaCl does not. Practice with these solids and tests will make these observations more recognizable. The part of the test students sometimes have the most trouble with is the written part. Here are some helpful tips.
Benedict’s Test Mechanism
Benedict’s solution (copper(II) sulfate) works on sugars that are aldose (aldehyde) sugars in a basic solution. The Cu2+ ion in the solution causes it to be blue, but it reacts with fructose or glucose to form Cu2O, which is a red precipitate.
Iodine Test for Starch The alpha –1 → 4 linkages between carbons in the starch produce the helical structure of the polysaccharide chain. The inner diameter of the helix is big enough for elementary iodine to become deposited, thus forming a blue complex (evidence for starch). When starch is mixed with iodine in water, an intensely colored starch/iodine complex is formed. Many of the details of the reaction are still unknown. But it seems that the iodine gets stuck in the coils of betaamylose molecules (beta-amylose is a soluble starch). The starch forces the iodine atoms into a linear arrangement in the central groove of the amylose coil. There is some transfer of charge between the starch and the iodine. That changes the way electrons are confined, and so, changes spacing of the energy levels. The iodine/starch complex has energy level spacings that absorb visible light— giving the complex its intense blue color.
Tips for Writing Net Equations
- Note the state of the materials involved in the reaction originally to determine how the substance shows up in the net equation. For example, solid sodium carbonate‘s reaction with a solution of hydrochloric acid would be different from solutions of hydrochloric acid and sodium carbonate’s reactions.
1st case: Na2CO3(s) + 2H3O+(aq) → 2Na+(aq) + CO2(g) + 3H2O(l)
2nd case: CO3 2-(aq) + 2H3O+(aq) → CO2(g) + 3H2O(l)
- Ionic solids in solution, as well as strong acids and bases, will be in “ion” form in the equations. Strong acids include HCl, HBr, HI, HNO3, HClO4, and H2SO4, and the strong bases include NaOH, KOH, Ca(OH)2, etc.
- Weak acids and bases (watch out for NH3) remain “intact” in net equations and precipitates are written as solids. Example. Reaction of vinegar with a solution of sodium carbonate: 2 HC2H3O2(aq) + CO3 2–(aq) → CO2(g) + H2O(l) + 2 C2H3O2 –(aq)
- Be sure to balance net equations just like any other equation.




