pH Meter
Buffer is a solution whose pH does not change very much when small amounts of acid (H+) or base (OH–) are added. This does not mean that no change occurs, only that it is small compared to the amount of acid or base added; the more acid or base added, the more the pH will change. Buffer solutions consist of a conjugate acid-base pair (weak acid plus its salt or weak base plus its salt) in approximately equal amounts (within a factor of 10). Thus, buffers work best at pH within 1 pH unit of the pKa. The concentration of a buffer refers to the total concentration of the acid plus the base form. The higher the concentration of the buffer, the greater its capacity to absorb acid or base. Most biological buffers are used in the range of 0.01–0.02 M concentration. The ratio of the 2 components and the pKa of the acid component determine the pH of the buffer.
| pH = pKa + log | [base form] |
| [acid form] |
If everything is behaving ideally, the pH should not depend on the buffer concentration or the presence of other ions in solution. In reality, some buffers do better at this than others. It’s best to check the pH of the final solution when preparing buffers from concentrated stocks.
Temperature will also affect pH since pKa values, like other equilibrium constants, change with temperature. Again, it’s best to check the pH of the buffer at the temperature it will be used.
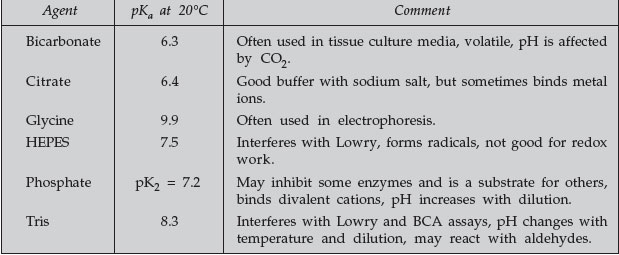
Some common buffers are listed in Table 1.
TABLE 1 Some Common Buffers |
 |
pH meters should be calibrated regularly using commercially available reference buffers.
LIST a protocol for making 1 liter of phosphate-buffered saline (PBS, 0.15 M NaCl, .02 M phosphate, pH = 7.2).
What is the pH of a 0.00043 N solution of HCl?
Weak Acid (Dissociation is Incomplete)
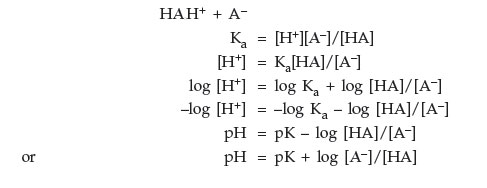
Henderson-Hasselbach Equation
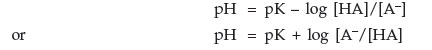
Definition. A buffer is a mixture of a weak acid and its salt (or a mixture of a weak base and its salt).
pH Meter and pH Electrode
The most commonly used electrode is made from borosilicate glass, which is permeable to H+, but not to other cations or anions. Inside is a 0.1 M HCl solution; outside there is a lower H+ concentration; thus the passage of H+ from inside to the outside. This leaves negative ion behind, which generates an electric potential across the membrane.
E = 2.3 × RT/F × log [H+]1/[H+]2
where,
R = gas constant,
T = absolute temperature,
F = Faraday constant
[H+]1 and [H+]2 are the molar H+ concentrations inside and outside the glass electrode.
A reference electrode (pH-independent and impermeable to H+ ions) is connected to the measuring electrode. Reference electrode contains Hg-Hg2Cl2 (calomel) paste in saturated KCl.
The concentration of 0.1 M HCl (inside the measuring electrode) may decrease by repeated use—therefore the pH meter has to be standardized against a solution of known pH.
pH Meter: A pH meter measures the voltage between electrodes placed in a solution.
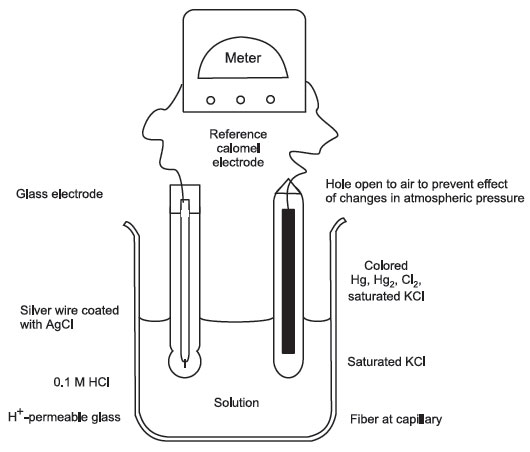 |
FIGURE 2 Glass and reference electrodes of a pH meter |




