Spectrophotometry
where,
A = ebc
e = extinction coefficient (a proportionality constant that dependsn on the absorbing species)
b = pathlength of the cuvette. Most standard cuvettes have a 1-cm path and, thus, this can be ignored
c = concentration.
A spectrophotometer or calorimeter makes use of the transmission of light through a solution to determine the concentration of a solute within the solution. A spectrophotometer differs from a calorimeter in the manner in which light is separated into its component wavelengths. A spectrophotometer uses a prism to separate light and a calorimeter uses filters.
Both are based on a simple design, passing light of a known wavelength through a sample and measuring the amount of light energy that is transmitted. This is accomplished by placing a photocell on the other side of the sample. All molecules absorb radiant energy at one wavelength of another. Those that absorb energy from within the visible spectrum are known as pigments. Proteins and nucleic acids absorb light in the ultraviolet range. The following figure demonstrates the radiant energy spectrum with an indication of molecules, which absorb in various regions of that spectrum.
The design of the single-beam spectrophotometer involves a light source, a prism, a sample holder, and a photocell. Connected to each are the appropriate electrical or mechanical systems to control the illuminating intensity, the wavelength, and conversion of energy received at the photocell into a voltage fluctuation. The voltage fluctuation is then displayed on a meter scale, is displayed digitally, or is recorded via connection to a computer for later investigation.
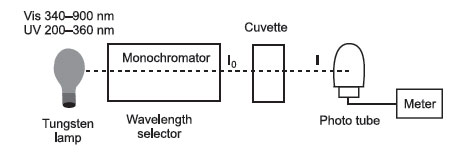 |
Figure 1: |
Spectrophotometers are useful because of the relation of intensity of color in a sample and its relation to the amount of solute within the sample. For example, if you use a solution of red food coloring in water, and measure the amount of blue light absorbed when it passes through the solution, a measurable voltage fluctuation can be induced in a photocell on the opposite side. If the solution of red dye is now diluted in half by the addition of water, the color will be approximately ½ as intense and the voltage generated on the photocell will be approximately half as great. Thus, there is a relationship between voltage and amount of dye in the sample.
Given the geometry of a spectrophotometer, what is actually measured at the photocell is the amount of light energy which arrives at the cell. The voltage meter is reading the amount of light transmitted to the photocell.
We can monitor the transmission level and convert it to a percentage of the amount transmitted when no dye is present. Thus, if ½ the light is transmitted, we can say that the solution has a 50% transmittance.
Transmittance is the relative percentage of light passed through the sample.
The conversion of that information from a percentage transmittance to an inverse log function known as the absorbance (or optical density).
The monochromator selects a particular wavelength. The sample and a blank are located in cuvettes. The light from the lamp passes through the cuvette and hits the phototube. The meter then records the signal from the phototube.
I0 = incident light, has intensity I0
I = light coming out of the cuvette (that contains light-absorbing substance), has intensity I.
Quantitative Aspects of Light Absorption: The Lambert-Beer Law
Transmittance, T, is the amount of light that passes through a substance. It is sometimes called percent transmission:
T = I/I0
% T = I/I0
I0 is the intensity of the incident light and I is the transmitted light. The light absorbed by the substance at a particular wavelength depends on the length of the light path through the substance. The negative logarithm of the transmittance, the absorbance A, is directly proportional to the amount of light absorbed and the length of the light path, and is described by the Lambert Law:
–log T = –log I/I0 = A = Kd
where d is the length of the solution in the cell and K is a constant.
The negative log of the transmittance is also directly proportional to the concentration of the absorbing substance, c, and is described by Beer’s Law:
–log I/I0 = –log T = A = Kc –log T = A = Edc
where E is a physical constant for a light-absorbing substance.
A = Ecd, d is usually 1 cm
A = absorbance (sometimes called the optical density)
E = molar extinction coefficient
c = concentration of the light-absorbing substance
Method
- Turn on the spectrophotometer and allow 10 minutes for the instrument to warm up before use.
- Adjust the wavelength to that specified for the procedure you are using.
- Be sure the cover is closed on the cuvette holder and use the left knob on the front panel to adjust the dark current so that the meter is reading 0 transmittance. At this point, you are simply adjusting the internal electronics of the instrument to blank out any residual currents. This adjusts the lower limit of measurements. It establishes that no light is equivalent to 0 transmittance or infinite absorbance.
- Insert a clean cuvette containing the blank into the holder. Be sure that the tube is clean, free of fingerprints, and that the painted line marker on the tube is aligned with the mark on the tube holder. Close the top of the tube holder. The blank for this exercise is the solution containing no dopachrome, but all other chemicals. The amount of solution placed in the cuvette is not important, but is usually about 5 mL. It should approximately reach the bottom of the logo printed on the side of the cuvette.
- Adjust the meter to read 100% transmittance, using the right knob on the front of the instrument. This adjusts the instrument to read the upper limit of the measurements and establishes that your blank will produce a reading of 100% transmittance (0 absorbance).
- Remove the blank from the instrument and recheck that your 0 transmittance value has not changed. If it does, wait a few minutes for the instrument to stabilize and read steps 1–5. Periodically throughout the exercise, check that the calibration of the instrument is stable by reinserting the blank and checking that the 0 and 100% T values are maintained.
- To read a sample, simply insert a cuvette holding your test solution and close the cover. Read the transmittance value directly on the scale.
- Record the percent transmittance of your solution, remove the test tube cuvette, and continue to read and record any other solutions you may have.
It is possible to read the absorbance directly, but with an analog meter (as opposed to a digital readout), absorbance estimations are less accurate and more difficult than reading transmittance. Absorbance can be easily calculated from the transmittance value. Be sure that you note which value you measure!
Absorption Spectrum
Analysis of pigments often requires a slightly different use of the spectrophotometer. In the use of the instrument for determination of concentration (Beer- Lambert Law), the wavelength was preset and left at a single value throughout the use of the instrument. This value is often given by the procedure being employed, but can be determined by an analysis of the absorption of a solution as the wavelength is varied.
The easiest means of accomplishing this is to use either a dual-beam spectrophotometer or a computer-controlled instrument. In either event, the baseline must be continuously reread as the wavelength is altered.
To use a single-beam spectrophotometer, the machine is adjusted to 0 first, with the blank solution, and then the sample is inserted and read. The wavelength is then adjusted up or down by some determined interval, the 0 is checked, the blank reinserted and adjusted, and the sample reinserted and read. This procedure continues until all wavelengths to be scanned have been read.
In this procedure, the sample remains the same, but the wavelength is adjusted. Compounds have differing absorption coefficients for each wavelength. Thus, each time the wavelength is altered, the instrument must be recalibrated.
A dual-beam spectrophotometer divides the light into 2 paths. One beam is used to pass through a blank, while the remaining beam passes through the sample. Thus, the machine can monitor the difference between the 2 as the wavelength is altered. These instruments usually come with a motor-driven mechanism for altering the wavelength or scanning the sample.
The newer version of this procedure is the use of an instrument, which scans a blank and places the digitized information in its computer memory. It then rescans a sample and compares the information from the sample scan to the information obtained from the blank scan. Since the information is digitized (as opposed to an analog meter reading), manipulation of the data is possible. These instruments usually have direct ports for connection to personal computers, and often have built-in temperature controls as well. This latter option would allow measurement of changes in absorption due to temperature changes (known as hyperchromicity). These, in turn, can be used to monitor viscosity changes, which are related to the degree of molecular polymerization with the sample. For instruments with this capability, the voltage meter scale has given way to a CRT display, complete with graphics and built-in functions for statistical analysis.
A temperature-controlled UV spectrophotometer capable of reading several samples at preprogrammed time intervals is invaluable for enzyme kinetic analysis. An example of this type of instrument is the Beckman DU-70.




