A General and Reliable Method for Obtaining High-Yield Metaphasic Preparations from Adherent Cell Lines:
Rapid Verification of Cell Chromosomal Content
In vitro studies are becoming more and more frequent in numerous domains of cell biology. For such purposes, a great variety of cell lines have been established either from normal tissues or, more frequently, from tumours. Very few of these cells present a normal karyotype: they are generally hyperdiploid and often contain rearranged chromosomes. Moreover, depending on the length of time in culture and on the culture conditions, populations of these cell lines can change and drift. Therefore, some general culture rules have to be respected (Ian Freshney, 1987), and it is highly recommended to avoid working with cells maintained in culture for a large number of generations.
Because of possible drift, cell lines need to be followed and controlled, with one fundamental control being the stability of their chromosomal content. Unfortunately, such control is not systematic. Moreover, in the case of new cell lines, it is frequent that their phenotype is described in detail with little or no information on their karyotype. It should be noted that even if karyotyping methods have long been described in the literature (for the history of human cytogenetics, see Jeening Lawce and Brown, 1997), it is not always easy to obtain metaphasic preparations from some cell lines. We were confronted with such a situation for some hybrid lines, in particular polarized rat hepatoma-human fibroblast WIF clones (Cassio et al., 1991; Shanks et al., 1994). This article describes a new method for obtaining, at a high yield, metaphasic preparations from delicate cell lines. This method is easy and reliable and has been applied successfully by many people to more than 50 different cell lines from different species.
This method is a combination of the two methods described by Worton and Duff (1979), the suspension method generally used for nonadherent and adherent cells and the "in situ" method that has been specifically developped for adherent cells (Cox et al., 1974; Peakman et al., 1977). When we tried to prepare metaphases from the well-polarized hybrid cell line WIF-B (Shanks et al., 1994), we only succeeded with the "'in situ" one. We performed many assays using the suspension method and they all failed: from two to three petri dishes containing 106 cells/dish in exponential phase, we obtained, at the best, a dozen metaphases (yield <10-5). In contrast, with the in situ method, the metaphase yield was very good and attained 1.5% of the total cell number (the maximal expected value for cells with a generation time of 2.5 days).
In the suspension method, mitoses are detached (mechanically or by proteolysis), whereas in the in situ method, mitoses stay in place. As polarized WIF-B cells have a highly differentiated plasma membrane, organized in different domains, we hypothesized that mitotic cells from this line are very fragile and are lost during or after detachment (may be during centrifugation). Therefore, to isolate metaphases from this line, a new method was developped that avoids cell detachment, at least during the early steps. The first steps of this method (metaphase arrest, hypotonic swelling, and first fixation) are performed in situ and it is only after the first fixation that cells are detached.
This method presents several advantages. First, it is applicable to every adherent cell line and does not depend on the adherent properties of the mitosis, as all mitoses (floating and adherent ones) are collected. Second, as with the in situ method, the yield in metaphases is very good (Fig. 1A) and therefore the metaphasic preparations are representative of the whole cell population. Third, this method requires fewer cells and is less wasteful in cells than the suspension method, an advantage for slow-growing cell lines where mitotic cells are rare or for cells where the results of karyotyping are required as soon as possible. Finally, as with the suspension method, the quality of the metaphase spreading is good (Fig. 1B) and can be adjusted, whereas with the in situ method, which allows only one attempt per culture, the spreading cannot be controlled and is very sensitive to cell overcrowding. Moreover, the chromosomes obtained by this new method are quite suitable for G banding, Giemsa 11 staining (Buys et al., 1984) (Fig. 1C), and fluorescent in situ hybridization (FISH) (Fig. 1D).
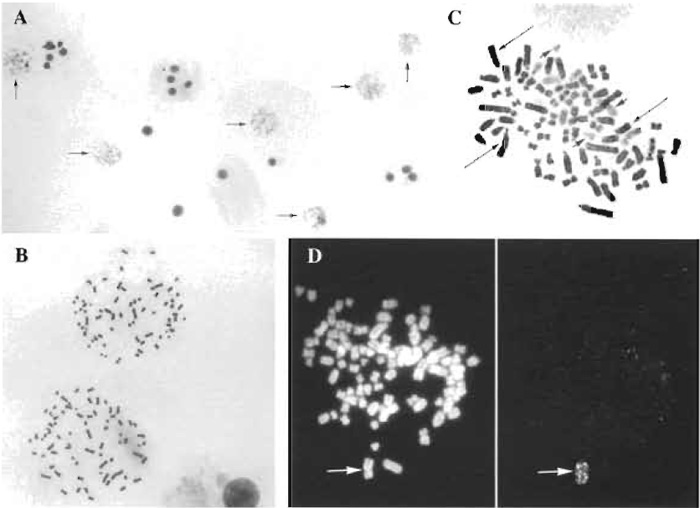 |
| FIGURE 1 Metaphases from HT29 and hybrid WIF lines. (A) HT-29 cells at low magnification; metaphases were obtained at a high yield as attested by the presence of six metaphases (arrows) among some 30 cells. (B) HT-29 metaphases at a higher magnification to illustrate chromosome spreading. (C) Detection of human (pale staining, small arrows) and rat (dark staining, long arrows) chromosomes by Giemsa 11 staining in a metaphase of the human x rat hybrid WIF-B. (D) Detection by FISH (right) of one copy of the human chromosome 2 (arrow) in a WIF 12-E metaphase that was counterstained with propidium bromide to show all the chromosomes (left). |
Growth medium (available from local suppliers)
Colcemid (10µg/ml; GIBCO, Cat. No. 2465)
Gurr buffer tablets, pH 6.8 (BDH, Cat. No. 33199)
Giemsa solution (Merck, Cat. No. 1.09204.0100)
10-cm tissue culture dishes (Falcon, Cat. No. 3003)
15-ml tubes (Falcon, Cat. No. 352099)
Precleaned, ground edge, microslides (ESCO, Cat. No. 2951R)
Glass jars
A low-speed centrifuge with a swinging-backet rotor for 15-ml tubes (as the IEC clinical centrifuge) is needed for harvesting cells, and a phase-contrast microscope is needed for examining cells and slides.
III. PROCEDURES A. Cleaning and Frosting of Microscope Slides
Steps
- Dip microscope slides in a jar containing 100ml methanol plus two to three drops of concentrated HCl for 12-24 h.
- Dry the slides one by one, place them in an appropriate jar, and freeze them at -20°C for at least 4h and at the most 7 days.
B. Optimization of Growth
Step
Grow cells in 10-cm Petri dishes in appropriate medium so that cells will be in midexponential phase with many mitoses on the day of harvest. Renew the dishes with 10ml of medium the day before the harvest.
C. Metaphase Arrest
Step
Add 0.2ml of colcemid for 1 h at 37°C to the dish containing the maximal number of mitotic cells. The other dishes can be used later if necessary.
Solutions
- Hypotonic solution (0.075M KCl): add H2O to 0.56g KCl to make 100 ml
- Fixative: Make fresh 3:1 methanol:glacial acetic acid; keep it at -20°C in an appropriate closed glass vessel and transfer to 4°C just before use.
Steps
- Collect the culture medium of the Petri dish in a 15-ml tube and centrifuge at room temperature, at approximatively 1000rpm (200g) for 5min, to collect the floating mitoses.
- Immediately after starting centrifugation, add 5ml of warm (37°C) hypotonic solution to the cells in the petri dish.
- Incubate the cells in the hypotonic solution at 37°C.
- As soon as the centrifuge stops, aspirate and discard the supernatant and add 1 ml of warm hypotonic solution to the pellet (depending on the cell line this pellet could be well visible or almost nonexistent). Resuspend the pellet rapidly by pipetting and add this suspension to the cells in the Petri dish, which now contain all the starting cells. Incubate at 37°C for a further 20-30 min.
E. Fixation Steps
- Add very carefully to the petri dish 6ml of cold (4°C) fixative with a glass pipette and place the dish on ice for 10 min.
- Detach the cells from the dish with a glass Pasteur pipette by repeated pipetting. Depending on the cell line, this step could be very easy or more laborious. Control the detachment under the microscope. If the cells are very sticky, try gentle use of a cell scraper.
- Transfer the cold cell suspension to a cold (4°C) 15-ml tube.
- Centrifuge at approximatively 1000rpm (200g) for at least 5 min.
- Remove all but about 0.2ml of supernatant and resuspend gently by shaking cell pellet in this small volume (no pipetting). Add 1 ml of cold fixative slowly and then another 4ml of cold fixative and leave on ice for at least 5 min.
- Repeat steps 4 and 5 (with a smaller volume of fixative) twice; the fixative can be added more quickly on the last fixations. At this point preparations can be stored overnight at 4°C or slides can be made immediately.
F. Spreading and Air Drying
Spreading is done by air drying onto frozen slides.
Steps
- Centrifuge the fixed cells at approximatively 1000rpm (200g) for at least 5min.
- Remove the supematant and gently resuspend the pellet in about 0.5 ml of cold fixative (make fresh fixative if cells have been stored overnight).
- Using a Pasteur pipette, drop two to three drops of the suspension at the top of a frozen slide inclined 20-30° from vertical. Let the drops run down the length of the slide as they spread. Wipe excess liquid from the underside of slide and let it dry at room temperature.
- Check this first slide under phase contrast so that adjustments can be made on subsequent slides if cells are too crowded or if the spreading is poor.
As the present technique was developed to verify the cell chromosomal content, we use Giemsa "solid staining" (Worton and Duff, 1979), which gives uniform staining of the chromosomes and makes it easy to count them. However, other staining methods can be applied (Fig. 1).
Solutions
- Gurr buffer, pH 6.8: Dissolve one Gurr buffer tablet, pH 6.8, in 1 liter H2O
- 5% Giemsa: Add 5ml of Giemsa to 95ml of Gurr buffer (or 10mM phosphate pH 6.8)
Steps
- Dip the slides in 5% Giemsa at room temperature for 15 min.
- Rinse twice with water and let dry at room temperature
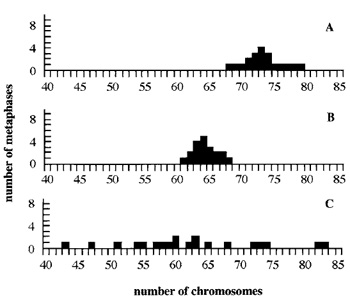 |
| FIGURE 2 Chromosomal content of HeLa cells. Metaphases were prepared from HeLa cells (ATCC collection) cultured routinely in three different laboratories (A-C). The chromosomal content of each cell population is different, and in all cases the chromosome number is lower than expected (published mean chromosome number 82, range 70-86). Note the very heterogeneous karyotype of HeLa cells in C. These results show how cells of a same line can change and drift. |
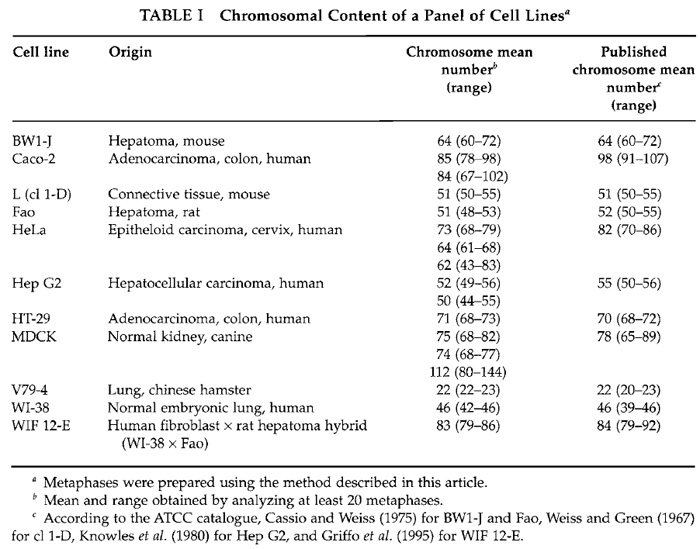
Using this method, metaphasic preparations from of a panel of well-known and frequently used lines, including polarized lines (Caco-2, MDCK, HT-29) and hepatic ones, were isolated and analyzed (Table I). Some lines (BW1-J, cl l-D, HT-29) displayed a chromosomal content similar or very near to that published previously, but the mean number of chromosomes, as the range, of other lines (Caco-2, HeLa) differed greatly from those published. Morever, in some cases, big differences were observed from the same line obtained from different laboratories. This was the case for HeLa cells (Fig. 2) and, to a lesser extent, for MDCK. In this latter case, one population over the three tested was very heterogeneous and greatly differed from the two others. Although both L and HeLa lines were established a long time ago (in 1940 and 1951, respectively) and thus cultured for a very large number of generations, the first line seems to be very stable, whereas the second one has considerable drift.
 |
- Use only glass pipettes for adding the fixative and for detaching cells after the first fixation. Fluffy and fuzzy chromosomes are obtained using plastic pipettes.
- Well-adherent cells can be detached by gentle pipetting at the end of the hypotonic shock before fixation.
- The presence of a thin layer of cytoplasm embedding the chromosomes can be avoided by performing additional fixations and by improving the spreading (increase the distance between the drop and the slide and adjust the angle of the slide).
Acknowledgments
I thank C. Delagebeaudeuf for pushing me to develop this method, C. Hamon-Benais, and V. Bender for the illustrations, and L. Sperling for careful reading of the manuscript. This work was supported in part by the Curie Institute (PIC Signalisation Cellulaire Grant 914), CNRS, and INSERM (contrat Prisme 98-09).
References
Buys, C. H. C. M., Aanstoot, G. H., and Nienhaus, A. J. (1984). The Giemsa 11 technique for species-specific chromosome differentiation. Histochemistry 81, 465-468.
Cassio, D., and Weiss, M. C. (1979). Expression of fetal and neonatal hepatic functions by mouse hepatoma-rat hepatoma hybrids. Somat. Cell Genet. 5, 719-738.
Cox, D. M., Niewczas-Late, V., Riffel, M. I., and Hamerton, J. L. (1974). Chromosomal mosaicism in diagnostic amniotic fluid cell cultures. Pediatr. Res. 8, 679-683.
Griffo, G., Hamon-Benais, C., Angrand, P. O., Fox, M., West, L., Lecoq, O., Povey, S., Cassio, D., and Weiss, M. C. (1993). HNF4 and HNF1, as well as a panel of hepatic functions are extinguished and reexpressed in parallel in chromosomally reduced rat hepatoma-human fibroblast hybrids. J. Cell Biol. 121, 887-898.
Ian Freshney, R. I. (1987). Culture of animal cells. In "A Manual of Basic Technique," 2nd Ed. A. R. Liss, New York.
Jennings Lawce, H., and Brown, M. G. (1997). Cytogenetics: An overwiew. In "The AGT Cytogenetics Laboratory Manual" (M. J. Barch, T. Knutsen, J. L. Spurbeck, eds.), 3rd Ed., pp. 19-50, Lippincott- Raven, Philadelphia.
Peakman, D. C., Moreton, M. F., and Robinson, A. (1977). Prenatal diagnosis: Techniques used to help in ruling out maternal cell contamination. J. Med. Genet. 14, 37-39.
Shanks, M. R., Cassio, D., Lecoq, O., and Hubbard A. L. (1994). An improved polarized rat hepatoma hybrid cell line. J. Cell Sci. 107, 813-825.
Weiss, M. C., and Green, H. (1967). Human-mouse hybrid cell line containing partial complements of human chromosomes and functioning genes. Proc. Nat Acad. Sci. USA 58, 1104-1111.
Worton, R. G., and Duff, C. (1979). Karyotyping. Methods Enzymol. 58, 322-344.
Suggested Reading
Barch, M. J., Knutsen, T., and Spurbeck, J. L. (ed.) (1997). "The AGT Cytogenetics Laboratory Manual," 3rd Ed. Lippincott-Raven, Philadelphia.




