Cygnets' Intracellular Guanosine 3',5'- Cyclic Monophosphate Sensing in Primary Cells Using Fluorescence Energy Transfer
Guanosine 3',5'-cyclic monophosphate (cGMP) is a key player in the regulation of various physiological processes, including smooth muscle tone, neuronal excitability, epithelial electrolyte transport, phototransduction in the retina, and cell adhesion (Eigenthaler et al., 1999; Kaupp and Seifert, 2002; Lincoln et al., 2001; Schlossmann et al., 2003). Although the cGMP second messenger pathway has been steadily gaining recognition for its role in intracellular signaling, cGMP remains the least well understood member of the cyclic nucleotide family due to the following peculiarities of the cGMP signal transduction system. (1) The control of cGMP levels is complex, with formation of cGMP occurring through two different forms of guanylyl cyclases (Russwurm and Koesling, 2002; Wedel and Garbers, 2001) and degradation by a number of cGMP-specific phosphodiesterases (PDEs) (Rybalkin et al., 2003). (2) The intracellular actions of cGMP are mediated by cGMP-dependent protein kinases (PKGs) (Hofmann et al., 2000) and by several types of cyclic nucleotide-activated ion channels (Kaupp and Seifert, 2002). (3) The enzymes modulating cGMP levels are expressed differentially throughout mammalian tissues. Furthermore, a tightly controlled equilibrium of synthesis and breakdown generates highly flexible intracellular cGMP transients, contributing to the experimental and conceptual obstacles posed by the multiplicity in mechanisms of cGMP signaling and the difficulty of studying cGMP in broken cell preparations.
II. MATERIALS AND INSTRUMENTATION
The RFL-6 (CCL-192) established cell line is from American Type Culture Collection. The FuGENE 6 transfection reagent is from Roche (1814443). Cygnet- 2.1 DNA (pcDNA3.1(-)-Cygnet-2.1) is constructed as described previously (Honda et al., 2001).
cGMP (40732-48-7; reconstituted in distilled H2O) and fluorescence grade 8-(4-chlorophenylthio)guanosine- 3', 5'-cyclic monophosphate [8-pCPT-cGMP; 51239-26-0; reconstituted in dimethyl sulfoxide (DMSO)] are from BIOLOG Life Science Institute.
The following chemicals have been used to modulate intracellular cGMP levels: Nitric oxide donors S-nitrosoglutathione (GSNO; Calbiochem 487920, protect from light and reconstitute in cold distilled H2O free of divalent cations other than Ca2+; stock solution is stable for 2h at 4°C), N-(2-aminoethyl)-N-(2- hydroxy-2-nitrosohydrazino)-l,2-ethylenediamine [NOC-22, spermine NONOate; Calbiochem 567703; reconstitute in 0.1 N NaOH, ≥pH 10; t½ of NO release = 230min in phosphate-buffered saline (PBS), pH 7.4, 22°C] diethylamine NONOate (DEA NONOate; Calbiochem 292500, reconstitute in distilled H2O, t½ of NO release - 16 min in PBS, pH 7.4, 22°C), and (±)- S-nitroso-N-acetylpenicillamine (SNAP; Calbiochem 487910; protect from light and reconstitute in DMSO; tl/2 of NO release = 10 h) are reconstituted to 100× the final concentration of 100µM prior to use and stored on ice until needed.
Atrial [ANP (3-28), rat; 14-5-44A] and brain [BNP (1- 32), Rat; 14-5-11A] natriuretic peptides are from American Peptide Company. The C-type natriuretic peptide [CNP (6-22), human and porcine; 05-23-0310] is from Calbiochem. Peptides are reconstituted in distilled H2O to 100 µM stock solutions and stored at -20°C for several months until their use at a final concentration of 0.1-1 µM.
The fluorescence spectrometer F-4500 is from Hitachi. Quartz fluorescence cells (14-385-918A) for spectrophotometers and Dithiothreitol (DTT, 16568- 0050) are from Fisher Scientific. Dithiothreitol (DTT, 16568-0050),
A light-duty portable punch size XX (130010001) outfitted with a 0.5-in. round dye (Type O) is from Roper Whitney of Rockford, Inc. A Sylgard 184 silicone elastomer kit is from Dow Corning Corporation. Coverslips (22 × 22 mm, 1 thickness; 12-544-10) are acquired from Fisher Scientific.
An inverted Nikon Diaphot 200 microscope equipped with a Nikon Fluor 40/1.30 oil Ph4DL objective (Part 140010) is outfitted with an ORCA ER cooled charge-coupled device camera (Hamamatsu). Three filter wheels, one each for excitation, emission, and neutral density filters, and a shutter at the excitation filter wheel are controlled by Lambda 10-2 optical filter changers from Sutter Instruments. A lambda LS xenon arc lamp and power supply are also obtained from Sutter Instruments. The Cameleons 2 filter set (71007a) purchased from Chroma Technologies for dual emission consists of a D440/20x excitation filter, a 455DCLP dichroic, and two emission filters (D485/40m and D535/30m). Neutral density filters (0.1, 0.3, 0.5, 1, 2, 3) are also obtained from Chroma. Image acquisition is controlled by a computer loaded with Metamorph and Metafluor 4.64 software from Universal Imaging (Media, PA). A stage adaptor to hold 35-mm imaging dishes was constructed at the Instrumentation and Model Facility at the University of Vermont.
A. Cygnet Expression and Purification
Express recombinant Cygnet-2.1 protein in Spodoptera frugiperda (Sf9) cells using the Bac-to-Bac baculovirus system (GIBCO/BRL) and purify using cAMP-agarose as described earlier (Honda et al., 2001).
B. In Vitro cGMP Titration
Perform cGMP titrations of Cygnet-2.1 by adding 50nM of protein to a quartz fluorescence cell with buffer (50 mM KPO4, pH 6.8, 10 mM DTT, 10 mM benzamidine, and 5mM EDTA) for a final volume of 500 µl. Excite samples in a fluorescence spectrometer at 432nm and monitor emission intensities from 450 to 550nm. Plat the ratios of the 475 to 525 emission intensities against the concentration of cGMP added to the sample to generate a titration curve.
C. Cell Culture
The following cell types have been used successfully to express cygnets and monitor intracellular cGMP levels. Details on their preparation and handling are as follow.
Cells should be cultured according to the supplier. Briefly, grow RFL-6 cells in Ham's F12 medium supplemented with 20% fetal bovine serum at 37°C, 5% CO2. Subculture every 5-6 days with 0.25% trypsin at a ratio of 1:4 and plate on glass-bottom dishes for imaging. RFL-6 cells should not used beyond passage number eight. Routinely verify cGMP responses with cygnet-transfected RFL-6 cells, an established cell line known to respond with high cGMP levels upon stimulation (Ishii et al., 1991).
2. Rat Aortic Smooth Muscle Cells (RASMC)
Preparation and Culture of RASMC
- Euthanize mature female or male Sprague-Dawley rat (250-350g) by a lethal dose of pentobarbital sodium and exsanguination and remove thoracic aorta.
- Clean aorta of fat and connective tissue and slice into 1- to 2-mm rings with a sterile scalpel.
- With a scalpel, score a 60-mm cell culture dish to create a grid of three horizontal and three vertical lines, and embed each ring where two grooves intersect.
- To prevent rings from detaching from the bottom of the dish, carefully add DMEM containing 10% FBS, 50µg/ml gentamicin, and 2.5µg/ml amphoteracin B and incubate at 37°C in humidified 5% CO2. Replenish media every 2-3 days and smooth muscle cells will proliferate from the aortic explants within 1 week.
- When cells reach confluency between gridlines, remove artery sections and rinse plate with trypsin. Add 1 ml fresh trypsin and aspirate off, leaving only a small amount in the dish. Incubate at 37°C until cells have detached, less than 5 min.
- Resuspend cells in 2-3 ml supplemented media and transfer approximately 300 µl of cell suspension to glass portion only of glass-bottom imaging dishes.
- When cells have adhered to the coverslip, aspirate media and replace with 2ml fresh media in entire dish. These cells are henceforth referred to as passage one (P1) cells. Use only P1 cells in imaging experiments.
- After extracting aorta, place in isolation media consisting of DMEM, 20mM HEPES, 1 mg/ml BSA, 5µg/ml amphoteracin B, and 50µg/ml gentamicin.
- Clean aorta of fat and connective tissue and incubate in isolation media supplemented with 1 mg/ml elastase and 130 units/ml collagenase for 8min at 37°C.
- Rinse aorta to remove endothelial and other nonadherent cells, and remove tunicae adventitia as an everted tube.
- Mince the medial layer of the aorta and digest pieces in isolation media supplemented with 200 units/ml collagenase for 1 to 2h or until single cells are attained.
- Wash cells twice in isolation media and culture in DMEM, 10% FCS, and 50µg/ml gentamicin.
D. Preparation of Imaging Dishes
- Prepare glass-bottomed dishes for fluorescence imaging by punching 0.5-in. holes in the bottoms of 35-mm cell culture dishes with an industrial punch.
- Mix 1 part Sylgard 184 curing agent with 10 parts Sylgard 184 silicone elastomer (w/w) carefully to avoid introducing air bubbles.
- Pipette a thin line of Sylgard around the hole on the bottom of the dish. Place a coverslip over the ring of Sylgard and tamp down with a cotton-tipped applicator to seal.
- Allow Sylgard to cure overnight.
- Sterilize dishes in a sterile hood under ultraviolet light for at least 30min.
- Dishes may be treated with 0.1 mg/ml collagen to promote cell adherence to coverslips.
- Grow primary RASMC to 50-60% confluency on 35- mm glass-bottomed dishes.
- For each 35-mm dish, pipette 3µl FuGENE 6 directly into 200µL serum-free DMEM in a plastic 1.5-ml microfuge tube, minimizing the contact that FuGENE 6 has with the wall of the tube.
- Add 1 µg highly purified pcDNA3.1(-)-Cygnet-2.1 to the DMEM-FuGENE 6. Close the tube and mix by inversion.
- Allow DNA/lipid complexes to form at room temperature for 15 min.
- Without removing media from 35-mm dishes, add transfection mixture dropwise using a pipette. Distribute transfection mixture around dish by gently shaking dish side to side.
- Incubate RASMC at 37°C for 24 h and RFL-6 cells 48 h before imaging. Culture media may be replaced after 6 h of transfection, if desired. However, leaving cells in FuGENE until the time of imaging does not cause toxicity.
Dual-Emission Imaging Protocol Using Metafluor
- Open Metafluor software from Universal Imaging Corporation and select "New" on the toolbar to begin a new experiment.
- Select configure/configure acquisition and define wavelengths 1 and 2 as ECFP and EYFP, respectively, and the excitation wavelength for both as 440nm. The emission wavelengths for ECFP and EYFP must be defined as 480 and 535nm, respectively. Chose file/save protocol file to save these parameters. When starting Metafluor from now on, begin by selecting "Protocol" and then open a new experiment.
- Check the "Save Images" box in the control panel. As long as the box remains checked, every image acquired from now on will be saved as part of the file you are now prompted to name. Note: Each experiment is saved as an .inf file, which is composed of a .tif file of every image acquired during the experiment.
- To locate transfected cells, manually select the 440-nm excitation filter and open the shutter (if applicable) in the illumination control box found in the configure menu.
- Focus the transfected cell(s) of interest through the camera by selecting "Focus" on the control panel and the focusing screen will appear. Choose "Start Focusing" and your image will appear. Bring the image displayed on the monitor into focus and then click on "Stop Focusing." Selecting "Close" will bring you back to the experimental menu.
- Select "Acquire One" on the toolbar to get an idea of what your image will look like. Cygnettransfected cells should be visible in both 475 and 535 emission channels (Figs. 1A and 1B). Cells can be pseudocolored during at a later period during data analysis to correlate color hue with 475/535 emission ratio (Figs. 1C and 1D). If the cell is too bright (saturated pixels appear black) or too dim, the exposure time may need to be altered in the configure acquisition menu.
- To start the experiment, press "Acquire" on the control panel menu and images will be captured at intervals until "Pause" is pressed.
- To set the time lapse between image acquisitions to 10s, select "Timelapse" on the control panel menu and enter the desired time.
- Define regions on your image in which to monitor intensity values by selecting "Regions" on the toolbar. Select the circle tool and place a circle on a dark area of the image for background measurements. Then trace several regions (shape and number depending on cell) on the cell(s) of which you would like to monitor the fluorescence. Select "Close" to return to the experimental menu.
Note: The defined regions appear on one of the images, but fluorescence intensity data are collected from both wavelength channels in the same exact position defined on the cell. Graph 1 and Graph 2 plot the wavelength intensity value and ratio of (wavelength 1)/(wavelength 2) intensity values, respectively, against time. - As the program acquires images and plots data collected from the regions that you have defined, monitor the trace in Graph 2, the plot of the ECFP/EYFP ratio. Once you have established a stable baseline, pharmacological agents can be added and the event markers used to note when things were added (select "Events" on the toolbar to define and mark events). At the end of an experiment, a cell-permeable cGMP analog can be applied to verify indicator fidelity (Fig. 2A).
- To end the experiment, select "Close" on the control panel.
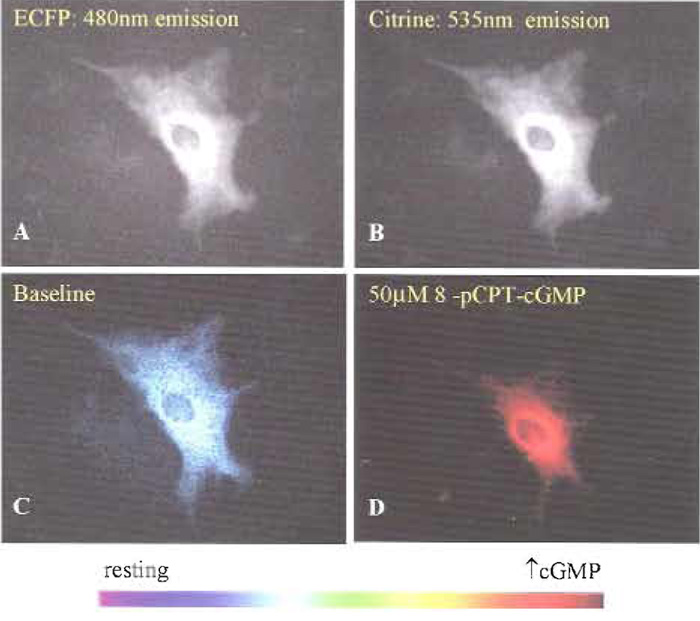 |
| FIGURE 1 Cygnet-2.1 expression indicates cytosolic localization and nuclear exclusion in cultured rat aortic smooth muscle cells (top) as shown by the fluorescence images of (A) ECFP (480nm emission) and (B) EYFPcitrine (535 nm emission). Pseudocolor representations of the 480- to 535-nm FRET ratio at resting (C) and elevated (D) cGMP levels were elicited with 50 µM 8-pCPT-cGMP. |
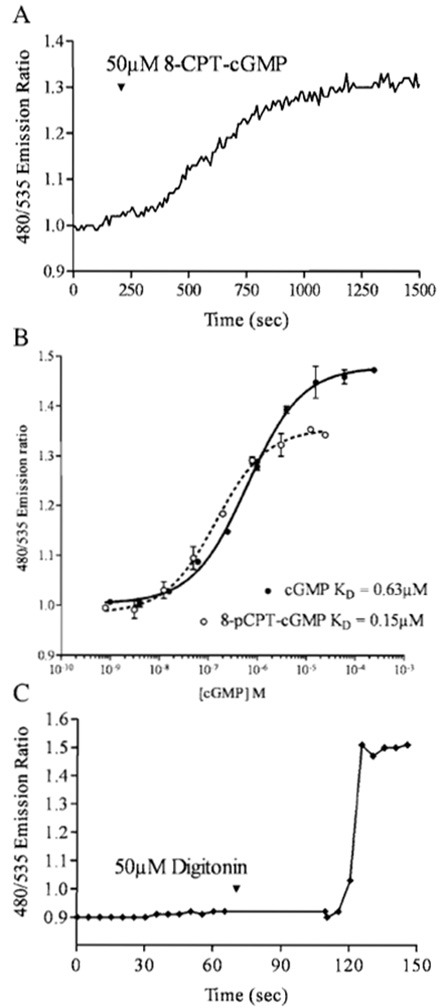 |
| FIGURE 2 (A) Cygnet-2.1 expressed in an individual RASM cell exhibits a 30% EYFP/citrine ratio change in response to a saturating dose of the membrane-permeable cGMP analog 8-CPT-cGMP. (B) In vitro Cygnet-2.1 titration with cGMP reveals a maximum FRET ratio change of 45%. Saturating concentrations of the analog 8-CPT-cGMP can only generate a 30% change. (C) A RASM cell expressing Cygnet- 2.1 was permeabilized with 50µM digitonin in an extracellular solution containing 8 µM cGMP. The indicator responded to the cGMP influx with a FRET ratio change of 47%. |
- To collect data, select "Open" from the toolbar and select an experiment.
- Define regions for background and on the cell(s) as before.
- To subtract the background (i.e., make the intensity of background equal to zero), select run experiment/reference images. In the pull-down menu, choose to subtract the average intensity of a region and indicate which number refers to the region defining the background. Check the box on the lower left-hand corner to "Subtract Background" and close the menu.
- Check "Log Data" on the control panel to save the intensity and ratio values of the wavelengths associated with each region.
- Press "Forward" on the control panel to run through the experiment. As each image appears, the intensity and ratio values for each region on each wavelength channel (ECFP and EYFP) are saved. To make certain that the regions stay in the approximate same place on the cell, their placement may need adjustment. The log file that has been generated can be opened in a spreadsheet program and time plotted against the ECFP/EYFP intensity ratio.
- To save individual images, select the desired image by playing through the experiment using the "Forward" button or the slider bar and then click on the image so that the window is highlighted. Under utilities/save as an 8-bit image, select to save the image as a .TIF file.
- To create a movie of a pseudocolored cell, define regions on the background and cell(s) and subtract background as described previously.
- Play through the experiment and determine the minimum and maximum ratio values the cell displays by referencing Graph 2.
- Select configure/image display control and choose Ratio 1 (ECFP/EYFP) from the top pull-down menu. From the lower pull-down menus choose intensity modulated display (IMD). In the "Minimum" and "Maximum" boxes, enter the approximate minimum and maximum ratio values determined from Graph 2. Setting the ratio values slightly inside the actual minimum and maximum values often enhances the visual effect as seen in the Ratio 1 box.
- After optimizing the color changes the cell undergoes throughout the experiment, check the "Save Ratio" box on the control panel and forward through the entire experiment. The images displayed in the Ratio 1 box have now been saved as .TIF files.
- Exit Metafluor and open Metamorph.
- Select file/build stack/numbered names and select the first image. When prompted, select the last image. A "stack" of .TIF files has now been created.
- Select stack/make movie, and a movie is generated from the compiled stack, which can be saved as an .AVI file under the stack menu.
- A representative movie corresponding to the trace shown in Fig. 3 is published as supplemental data on the Cell Biology website.
Note: .AVI files generated with Metamorph are very large (40-100MB) but can be compressed down to a few megabytes (50× or more) using a program such as VideoFramer.
At approximately 50-60% confluency, passage one cells were transfected with FuGENE 6 using a 3:1 ratio of FuGENE reagent to pcDNA3.1-Cygnet-2.1 DNA. Cells were imaged 24h posttransfection at 25°C using Hank's balanced salt solution with 20 mM HEPES (pH 7.35) and glucose (2g/liter) as the extracellular solution and 500-ms exposures at 10-s intervals.
Cygnet-2.1 expressed in both rat aortic smooth muscle cells (Figs. 1A and 1B) and RFL-6 cells (Honda et al., 2001) demonstrated cytosolic localization and nuclear exclusion when ECFP and citrine emissions were viewed individually. Pseudocoloring of the cell (Figs. 2C and 2D) correlates the ratio of the ECFP/citrine emissions to a color scale, with lower and higher ratios and cGMP levels represented by blue and red, respectively. A saturating dose of the cell membrane-permeable cGMP analog 8-pCPT-cGMP changed the pseudocoloring from blue to red (Fig. 1D) and correlates to a 30% increase in FRET ratio (Fig. 2A). However, in vitro results with purified Cygnet-2.1 showed that cGMP consistently causes a 40-50% ratio change. To analyze this apparent discrepancy, Cygnet- 2.1 purified from Sf9 cells was titrated with cGMP and 8-CPT-cGMP (Fig. 2B). The purified indicator's maximum ratio change attained with cGMP was approximately 45%, while the analog still only elicited a maximal ratio change of 30%. In fact, in cells permeabilized with 50µM digitonin in the presence of saturating concentrations of cGMP, cygnets demonstrated a 47% FRET ratio change as expected from the in vitro results (Fig. 2C).
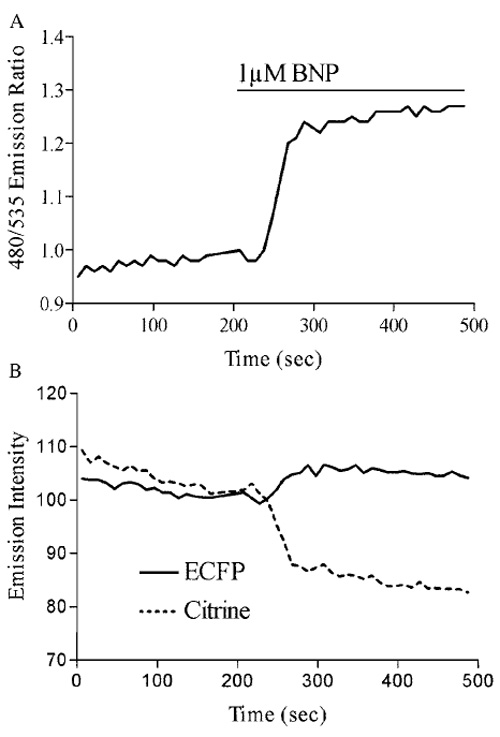 |
| FIGURE 3 (A) Stimulation of the particulate guanylate cyclase (pGC) NPR-A with the natriuretic peptide BNP produces a FRET ratio change of approximately 28%. (B) Analysis of the individual emission intensities of ECFP and citrine during pGC activation demonstrates an increase in ECFP and a decrease in citrine emissions. |
Experiments acquiring images at 10-s intervals can run for greater than 30min. We have accumulated as many as 200 exposures before applying a stimulus and have observed no significant loss in indicator sensitivity. It should be noted that cells have the tendency to react quite differently to the same stimulus.
V. PITFALLS
- Transfection efficiency and cygnet expression may be low in some cells, particularly in primary cultures. Optimal expression may require alternative transfection reagents and optimization of transfection conditions, depending on the cell type.
- Because intracellular cGMP fluctuations can potentially be buffered by the expression of the indicator, it is important to compare the total cGMP-binding capacity of untransfected and cygnettransfected cells. This can be accomplished by the use of antibodies to PKG to determine if total PKG immunoreactivity, and therefore cGMP-binding capacity, is elevated by the expression of cygnets.
- Because excessive illumination of cygnets may result in photobleaching of GFP mutants, fluorophore excitation should occur at the minimum intensity, frequency, and duration possible. The emission profiles of ECFP and citrine should be monitored to ascertain that changes in FRET are not due to aberrant behavior of the individual fluorophores due to photobleaching or other phenomena such as pH-induced alterations.
Acknowledgments
This work was supported by NSF Grant MCB-9983097 (WRGD) and the Totman Medical Research Trust.
Cornwell, T. L., and Lincoln, T. M. (1989). Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle ceils: Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J. Biol. Chem. 264, 1146-1155.
Eigenthaler, M., Lohmann, S. M., Walter, U., and Pilz, R. B. (1999). Signal transduction by cGMP-dependent protein kinases and their emerging roles in the regulation of cell adhesion and gene expression. Rev. Physiol. Biochem. Pharmacol. 135, 173-209.
Hofmann, E, Ammendola, A., and Schlossmann, J. (2000). Rising behind NO: cGMP-dependent protein kinases. J. Cell Sci. 113, 1671-1676.
Honda, A., Adams, S. R., Sawyer, C. L., Lev-Ram, V., Tsien, R. Y., and Dostmann, W. R. (2001). Spatiotemporal dynamics of guanosine 3',5'-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc. Natl. Acad. Sci. USA 98, 2437-2442.
Ishii, K., Sheng, H., Warner, T. D., Forstermann, U., and Murad, E (1991). A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am. J. Physiol. 261, H598-H603.
Kaupp, U. B., and Seifert, R. (2002). Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769-824.
Lincoln, T. M., Dey, N., and Sellak, H. (2001). cGMP-dependent protein kinase signaling mechanisms in smooth muscle: From the regulation of tone to gene expression. J. Appl. Physiol. 91,1421-1430.
Pfeifer, A., Ruth, P., Dostmann, W., Sausbier, M., Klatt, P., and Hofmann, E (1999). Structure and function of cGMP-dependent protein kinases. Rev. Physiol. Biochem. Pharmacol. 135, 105-149.
Russwurm, M., and Koesling, D. (2002). Isoforms of NO-sensitive guanylyl cyclase. Mol. Cell. Biochem. 230, 159-164.
Ruth, P., Landgraf, W., Keilbach, A., May, B., Egleme, C., and Hofmann, E (1991). The activation of expressed cGMPdependent protein kinase isozymes Iα and Iβ is determined by the different amino-termini. Eur. J. Biochem. 202, 1339-1344.
Rybalkin, S. D., Yan, C., Bornfeldt, K. E., and Beavo, J. A. (2003). Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ. Res. 93, 280-291.
Schlossmann, J., Feil, R., and Hofmann, E (2003). Signaling through NO and cGMP-dependent protein kinases. Ann. Med. 35, 21-27.
Smith, J. B., and Brock, T. A. (1983). Analysis of angiotensin stimulated sodium transport in cultured smooth muscle cells from rat aorta. J. Cell Physiol. 114, 284-290.
Wedel, B., and Garbers, D. (2001). The guanylyl cyclase family at Y2K. Annu. Rev. Physiol. 63, 215-233.
Zaccolo, M., and Pozzan, T. (2002). Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295, 1711-1715.
Zhao, J., Trewhella, J., Corbin, J., Francis, S., Mitchell, R., Brushia, R., and Walsh, D. (1997). Progressive cyclic nucleotide-induced conformational changes in the cGMP-dependent protein kinase studied by small angle X-ray scattering in solution. J. Biol. Chem. 272, 31929-31936.




