Neural Crest Stem Cells
Cellular diversity in the vertebrate peripheral nervous system is achieved by the differentiation of neural crest stem cells (NCSCs) in a spatially and temporally regulated fashion. During embryonic development, neural crest cells detach from the neuroepithelium of the dorsal neural tube and migrate to their sites of terminal differentiation (Le Douarin and Kalcheim, 1999). At least a subpopulation of these cells are multipotent and able to give rise to neuronal, glial, and nonneural derivatives, as has been shown by grafting experiments and by clonal analysis in culture and in vivo (Anderson et al., 1997; Ziller et al., 1983). Moreover, some crest cells display features of stem cells that not only generate multiple cell types, but also have the capacity to self-renew (Morrison et al., 1999; Stemple and Anderson, 1992). In migrating neural crest and in target tissues of the neural crest, multipotent crest cells coexist with cells that have a more restricted developmental potential (Sommer, 2001). Cell intrinsic differences between crest cells from different regions of the peripheral nervous system are involved in the generation of neural diversity. In addition, the decision of a NCSC to survive, self-renew, or differentiate depends on the combinatorial activity of multiple environmental signals (Sommer, 2001). To identify these signals, NCSCs have to be challenged by altering both their extracellular environment and their intrinsic genetic programs. This is facilitated greatly by the availability of neural crest culture systems. Because signals required to maintain undifferentiated, multipotent NCSCs for an extended period of time in culture have not yet been identified, various cell culture conditions have been established by different laboratories. This article describes methods for culturing rat and mouse neural crest stem cells, largely based on articles by Stemple and Anderson (1992) (rat NCSCs), Sommer et al. (1995) (mouse NCSCs), Greenwood et al. (1999) (culture conditions permissive for sensory neurogenesis), and Morrison et al. (1999) (postmigratory NCSCs).
A. Instruments and Plasticware
Modular incubator chamber (Billups-Rothenberg Inc., www.brincubator.com); Dumont #3 and #5 forceps (Fine Science Tools, Cat. Nos. 11231-30 and 11251-10); Vannas style iris spring scissors (Fine Science Tools, Cat. No. 15000-02); cell culture dishes, 35 × 10-mm style (Corning Cat. No. 430165); 6-well cell culture dishes (Corning Cat. No. 430166); tissue culture dishes, 60 × 15-mm style (Nunclon DSI Cat. No. 064194); Omnifix, 50ml (Braun Cat. No. 459785OF); Millex syringe-driven filter unit, 0.22µm (Millipore Cat. No. SLGPO33RB); and Steritop 500 GP express plus membrane, 0.22µm (Milipore Cat. No. SCGPT05RE).
B. General Buffers and Reagents
Hanks' balanced salts (HBSS) without Ca2+ and Mg2+ 95.18g (Amimed Cat. No. 3-02P30-M), Hanks' balanced salts without phenol red, 97.5g (Amimed Cat. No. 3-02P32-M), phosphate-buffered saline (PBS) Dulbecco (D-PBS) 501 (Biochrom KG Cat. No. L-182-50); formaldehyde solution, 250ml (Fluka Cat. No. 47608); and potassium hydroxide (Fluka Cat. No. 60375).
Dispase 1 (neutral protease) 10 × 5mg (Roche Cat. No. 1 284 908); collagenase types 1, 3, and 4 (Worthington Biochemical Coorporation); 0.25% trypsin-EDTA, 100 ml (Invitrogen-GIBCO Cat. No. 25200-056); 2.5% trypsin (10×), 100ml (Invitrogen- GIBCO Cat. No. 25090-028); hyaluronidase type IV-S, 50mg (Sigma H-4272); and deoxyribonuclease type 1 (Sigma Cat. No. D-4263).
E. Media Components
Dulbecco's modified Eagle medium (DMEM)-low glucose, 500 ml (Invitrogen-GIBCO Cat. No. 11880-028); DMEM, 500ml (Invitrogen-GIBCO Cat. No. 41966- 029); minimum essential medium (MEM), 500ml (Invitrogen-GIBCO Cat. No. 31095-029); Leibovitz's L15 medium powder (Invitrogen-GIBCO Cat. No. 41300- 021); dimethyl sulfoxide (DMSO) 251 (Aldrich Cat. No. 27,043-1); N2-supplement (100×), 5ml (Invitrogen- GIBCO Cat. No. 17502-048); 2-mercaptoethanol (Sigma Cat. No. M-7522); B-27 supplement (50×), 10 ml (Invitrogen-GIBCO Cat. No.17504-048); forskolin, 10 mg (Sigma Cat. No. F-6886); fetal bovine serum (FBS), 500ml (different suppliers); water, cell culture tested, 500ml (Sigma Cat. No. W-3500); phenol red solution, 100ml (Sigma Cat. No. P-0290); imidazole, I g (Sigma Cat. No. 1-0250); hydrochloride acid solution, 100ml (Sigma Cat. No. H-9892); sodium bicarbonate, 500g (Sigma Cat. No. S-5761); dexamethasone, 25 mg (Sigma Cat. No. D-4902); bovine albumin crystalline, 5g (Sigma Cat. No. A-4919); 99.5% glycerol, 500ml (Invitrogen-GIBCO Cat. No. 15514-011); transferrin, holo, bovin plasma, 100mg (Calbiochem Cat. No. 616420); putrescine (Sigma Cat. No. P-7505); (+/-)-α- tocopherol (vitamin E), 5g (Sigma Cat. No. T-3251); insulin, 100mg (Sigma Cat. No. 1-6634); human epidermal growth factor (hEGF), 200µg (R&D Systems Cat. No. 236-EG-200); human nerve growth factor (β- NGF) (R&D Systems Cat. No. 256-GF-100); selenious acid (Aldrich Cat. No. 22,985-7); basic fibroblast growth factor (bFGF), 25µg (R&D Systems Cat. No. 233-FB-025); progesterone, I g (Sigma Cat. No. P-8783); human neurotrophin 3 (NT3), 10µg (BioConcept Cat. No. 1 10 01862); human brain-derived neurotrophic factor (BDNF), 10µg (BioConcept Cat. No. 1 10 11961); insulin-like growth factor (IGF1), 250 µg (R&D Systems Cat. No. 291-G1-250); and retinoic acid (Sigma Cat. No. R-2625).
Aspartic acid, 100g (Sigma Cat. No. A-4534); Lglutamic acid, 100g (Sigma Cat. No. G-8415); L-proline, 25g (Sigma Cat. No. P-4655); L-cystine (Sigma Cat. No. C-7602); p-aminobenzoic acid (Aldrich Cat. No. 42,976-7); 3-aminoproprionic acid, 100g (Sigma Cat. No. A-9920); vitamin B-12, I g (Sigma Cat. No. V- 6629); myo-inositol, 50 g (Sigma Cat. No. 1-7508); choline chloride, 100g (Sigma Cat. No. C-7527); fumaric acid, 100g (Sigma Cat. No. F-8509); coenzyme A, 100mg (Sigma Cat. No. C-4282); D-biotin (Sigma Cat. No. B- 4639); and DL-(X-lipoic acid, 5 g (Sigma Cat. No. T-1395).
G. 1:1:2
Dextrose [D-(+)-glucose] (Sigma Cat. No. G-7021); L-glutamine (Sigma Cat. No. G-6392); and penicillin-streptomycin, 100 ml (Invitrogen-GIBCO Cat. No. 15140-015).
H. Mix7
DL-β-Hydroxybutyric acid sodium salt (Sigma Cat. No. H-6501); cobalt chloride, 25 g (Sigma Cat. No. C-8661); oleic acid, 250mg (Sigma Cat. No. O-7501); α-melanocyte-stimulating hormone (α-MSH), 5mg (Sigma Cat. No. M-4135); prostaglandinE1, 1 mg (Sigma Cat. No. P-5515); and 3,3',5- triiodo L-thyronine (T3), 100mg (Sigma Cat. No. T-6397).
DMPH B grade (Calbiochem Cat. No. 31636); Lglutathione (Sigma Cat. No. G-6013); and L-ascorbic acid, 25 g (Sigma Cat. No. A-4544).
III. PROCEDURES
A. Solutions and Stocks 1. Chicken Embryo Extract (CEE)
Incubate white chicken eggs for 11 days at 38°C in a humidified atmosphere. Wash eggs with 70% ethanol, open the top of each shell, and remove the embryos and place them into a petri dish containing MEM at 4°C Macerate the embryos by pressing through a 50-ml syringe into a 50-ml centrifuge tube (Falcon) (approximately 25ml of homogenate per tube). Add 25ml of MEM per 25ml of chicken homogenate. Shake the tubes at 4°C for 1 h. Add 100 µ (800 U) sterile hyaluronidase to 50ml of chicken homogenate and centrifuge the mixture for 6h at 30,000 g at 4°C. Collect the supernatant, filtrate through a 0.22-µm Steritop filter, and distribute in 5-ml aliquots. Store at -80°C until use.
Collect 198ml water into a detergent-free beaker. To the 198ml water add 0.6g aspartic acid, 0.6g L-glutamic acid, 0.6g L-proline, 0.6g L-cystine, 0.2 g p-aminobenzoic acid, 0.2g 3-aminoproprionic acid, 80mg vitamin B-12, 0.4g myo-inositol, 0.4g choline chloride, 1.0g fumaric acid, and 16mg coenzyme A. Suspend 0.4 mg D-biotin and 100 mg DL-α-lipoic acid in 10ml water and add 2ml of this solution to the solution in the beaker. Mix the solutions, prepare 1.5-ml aliquots and store at-20°C until use.
3. L-15C02
Add 3.675g L-15 powder, 0.019g imidazole, and 1.6ml SVM to 288 ml water in a 500-ml beaker. Mix the solution until dissolved and add 240µl of 1M HCl to adjust the pH between 7.35 and 7.40. In a 100-ml beaker, mix 0.8g sodium bicarbonate, 120µl phenol red, and 59ml water. Apply CO2 directly to this solution using a Pasteur pipette until it turns yellow and no further color change can be observed. Then mix the sodium bicarbonate solution with the L-15 solution and apply CO2 again for a short time. Determine the pH, which should range between 7.15 and 7.25. Filter the solution through a 0.22-µm filter into a 500-ml tissue culture bottle and store at 4°C until use.
4. 1:1:2
Slowly dissolve 60g dextrose in 160ml water by stirring. Adjust the volume to 200ml after dextrose is dissolved. Add 100ml glutamine (200mM) and 100ml penicillin-streptomycin, filter the solution through a 0.22-µm filter, and distribute in 2-ml aliquots. Store at -20°C until use.
5. Fresh Vitamin Mix (FVM)
Dissolve 5mg DMPH, 25mg glutathione, and 500 mg L-ascorbic acid in 80 ml water by stirring. After all chemicals are dissolved, raise the pH to 5-6 with 1M potassium hydroxide. Then adjust the volume to 100ml, filter the solution through a 0.22 µm filter, and store in 550-µ aliquots at-20°C until use.
Dissolve 630 mg DL-β-hydroxybutyrate in 10 ml water (1000× stock). Dissolve cobalt chloride to 10mg/ ml in water and then add 25µl from that solution to 10ml of L15CO2 to obtain a stock of 25µg/ml (1000×). Dissolve biotin to 10mg/ml in DMSO and then dilute to 1 mg/ml in L15CO2 (1000×). Dissolve oleic acid to 2.8mg/ml in water and then add 37.5µl of this to 10ml of L15CO2 to 10µg/ml (1000×). Dissolve αMSH to 1 mg/ml in water and dilute to 0.1 mg/ml in L15CO2 (1000×). Dissolve prostaglandin to 1 mg/ml in 95% ethanol and dilute 1:100 in L15CO2 to 10µg/ml (1000×). Dissolve T3 to 10mg/ml in DMSO and add 67.5µl of this to 10ml L15CO2 to 67.5µg/ml (1000×). Add 5ml of each of the aforementioned solutions to 15 ml of L15CO2, filter the solution through a 0.22-µm filter, and store in 550-µl aliquots at-20°C until use.
7. Additives
Dissolve most additives in H20, except for the following: dissolve retinoic acid to 17.5mg/ml in DMSO and then dilute this solution 1:500 in equal volumes of 95% ethanol and L15CO2 to 35µg/ml (1000×). Dissolve vitamin E to 50mg/ml in DMSO and dilute this 1:10 to 5 mg/ml (1000×). Dissolve 3.93 mg dexamethasone in 10 ml 95% ethanol to 1 mM stock; for use, dilute stock 1 : 100 in L15CO2. Dissolve 50 mg insulin in 10 ml 5 mM HCl solution. Dissolve 31.5 mg progesterone in 10 ml 95% ethanol to 10 mM and dilute 1 : 100 in 95% ethanol to a 0.1 mM stock. Dissolve 100 mg transferrin in 2ml l× D-PBS. Dissolve 100~tg β-NGF in 2ml L15CO2(+1mg/ml BSA) to 50µg/ml. Dissolve 200µg hEGF in 2ml L15CO2(+1mg/ml BSA) to 100µg/ml. Dissolve 25µg bFGF in 1 ml L15CO2(+1 mg/ml BSA) to 25µg/ml. Dissolve 10µtg NT-3 in 400µl L15CO2 (+1 mg/ml BSA) to 25µg/ml. Dissolve 10µg BDNF in 400µl L15CO2(+1mg/ml BSA) to 25µg/ml. Dissolve 250µg IGF-1 in 2ml L15CO2(+1 mg/ml BSA) to 125µg/ ml. Store these additives at-80°C until use. Dissolve 80mg putrescine in 10ml water to 8mg/ml. Dissolve 1.29g selenious acid in 10ml water and dilute to a stock of 0.1 mM. Store these additives at 4°C until use.
1. Standard Medium (SM)
To prepare 50ml of defined medium (DM) (Stemple and Anderson, 1992) take 46.3ml L15CO2, 50mg BSA (1 mg/ml), 2ml 1:1:2, 500µl FVM, 315µl glycerol, 100µl putrescine (16 µg/ml), 100 µl transferrin (100 µg/ ml), 50µl vitamin E (5µg/ml), 50µl EGF (100ng/ml), 50µl insulin (5µg/ml), 20µl NGF (20ng/ml), 15µl selenious acid (30nM), 8µl bFGF (4ng/ml), 10µl progesterone (20nM), 0.5µl dexamethasone (100nM), 500µl Mix7. To prepare 50ml standard medium, add to 45ml DM 5ml CEE and 50µl retinoic acid (35 ng/ml). Filter the medium through a 0.22-µm filter and store at 4°C until use. (Final concentrations are in parentheses.)
A simplified SM has been used by Morrison and colleagues (Bixby et al., 2002; Morrison et al., 1999). To prepare 50ml of standard medium take 38.9ml DMEM low glucose, 2ml 1:1:2, 25µl retinoic acid (17.5ng/ml), 500µl N2 salt supplement (1%), 15µl selenious acid, 1 ml B27 supplement (1:50), 2.5µl 2- mercaptoethanol (50µM), 8µl IGF1 (20ng/ml), and 7.5ml CEE (15%). For SM1, add 40µl bFGF (20ng/ml). After 6 days of culture incubation, use SM2 to allow differentiation: reduce bFGF levels to 20µl (10ng/ml) and CEE to 500µl (1%) and fill up to 50ml with DMEM low glucose. Filter the medium through a 0.22-µm filter and store at 4°C until use.
Comment
In our hands, the simplified medium according to Morrison et al. (1999) was less efficient in supporting neural crest cultures than the more complex standard medium (Stemple and Anderson, 1992). To increase cell survival, IGF1 has been added in a more recent study by Bixby et al. (2002).
To prepare 50ml of SN medium (Greenwood et al., 1999) take 47.1ml L15CO2, 50mg BSA (1 mg/ml), 2ml 1:1:2, 50µl insulin (5µg/ml), 100µl putrescine (16µg/ml), 10µl progesterone (20nM), 15µl selenious acid (30nM), 0.5µl dexamethasone, 143µl glycerol, 50µl vitamin E (5µg/ml), and 500µl Mix7. To culture NCSCs for 2-3 days, make SN1 by adding 20µl bFGF (10ng/ml) to SN. For further differentiation, use SN2, which is prepared by adding the following reagents to SN: 8 µl bFGF (4ng/ml), 25 µl EGF (50ng/ml), 25µl retinoic acid (17.5ng/ml), 25µl NGF (25 ng/ml), 25 µl BDNF (12.5 ng/ml), 25 µl NT3 (12.5ng/ml), and 250µl CEE (0.05%). Filter the medium through a 0.22-µm filter and store at 4°C until use. In brackets, (Final concentrations are in parentheses.)
C. Isolation
1. Migratory Neural Crest Stem Cells from Neural Tube Explant Cultures
Mouse NCSCs are isolated at embryonic day 9 (E9) (Sommer et al., 1995), whereas rat NCSCs are isolated at E10.5 (Stemple and Anderson, 1992) (Fig. 1). The isolation of trunk neural crest is described here.
- Sacrifice time-mated females by CO2 asphyxiation in accordance with National Institutes of Health guidelines.
- Remove the uterus into a 10-cm petri dish containing sterile HBSS without phenol red.
- With a pair of fine spring scissors, cut an opening along the length of each uterus, being careful not to cut into the embryo and yolk sac.
- Under a dissecting microscope, remove the embryos by squeezing the uterus gently with a Dumont #3 forceps while cutting the surface of the decidua and amnionic sac with another #3 forceps. After every embryo has been removed from the uterus, transfer the embryos to a new 10-cm petri dish containing sterile HBSS without phenol red.
- Use an L-shaped electrolytically sharpened tungsten needle and a Dumont #5 forceps to dissect a block of tissue from a region corresponding to the region caudal to the heart to the most caudal somite. Pool and place the trunks into a new 3-cm petri dish. They can be stored for an hour at 4°C.
- Prepare digestion mix using 12 ml HBSS without Ca2+ and Mg2+ and one vial (5 mg) of dispase 1. Distribute digestion mix to three 3-cm petri dishes. Transfer the trunks with a Pasteur pipette to the dispase mix and transfer them from the first to the second and then from the second to the third petri dish. Triturate slowly for 2min at room temperature. Place the dish at 4°C for 6 min.
- Triturate the trunks gently and patiently until the neural tubes are free of other tissues. Transfer every tube to DMEM+10% FBS to stop the digestion reaction. Then transfer the tubes to the appropriate media.
- Coat 35-mm Corning tissue culture dishes with fibronectin (FN) as described later and preincubate with appropriate media. Withdraw media and plate three to four neural tubes directly onto the dish. Monitor each step carefully under a dissecting microscope.
- Allow the tubes to attach for 30min at 37°C in a 5% CO2 atmosphere and then flood the dish gently with 1 ml medium. If the tubes do not attach, withdraw the media gently and repeat the step until the tube is properly attached to the dish. Incubate the dishes in medium appropriate to the experiment.
2. Isolation of Postmigratory Neural Crest Stem Cells
Postmigratory NCSCs from dorsal root ganglia (DRG) (Fig. 1), from sciatic nerve, and from gut have been isolated at various stages of development, both from mouse and from rat embryos (Bixby et al., 2002; Hagedorn et al., 1999; Lo and Anderson, 1995; Morrison et al., 1999; Paratore et al., 2002; Pomeranz et al., 1993). Studies have also shown that NCSCs can be isolated from postnatal and adult gut (Kruger et al., 2002). Note that the plating efficiency is very low for adult gut NCSCs.
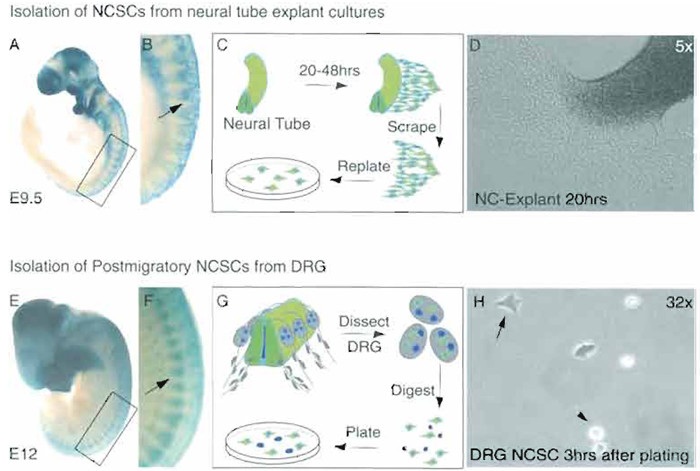 |
| FIGURE 1 To illustrate the localization of migratory and postmigratory neural crest cells (arrows), X-gal staining was performed on mouse embryos at E9.5 (A) and at E12 (E), in which neural crest cells had been marked by WntlCre-mediated recombination (Danielian et al., 1998) of the ROSA26 reporter gene (Soriano, 1999). Boxes in A and E represent areas enlarged in B and F, respectively. (C) Scheme of the explant culture system of a trunk neural crest and (D) phase-contrast picture of a neural crest explant at 5x magnification. (G) Isolation scheme of postmigratory NCSCs from DRG and (H) phase-contrast picture of DRG-derived postmigratory NCSCs (arrow) and neuronal cells (arrowhead). |
- Sacrifice time-mated females as described. After removal from the uterus, transfer the embryos to a new 10-cm petri dish containing sterile HBSS without phenol red.
- Use an L-shaped electrolytically sharpened tungsten needle and a Dumont #5 forceps to dissect a block of tissue from a region rostral to the heart to the most caudal somite. Pool and place the trunks into a new 3-cm petri dish. They can be stored for an hour at 4°C.
- Gently drive the tungsten needle between the cartilage primordium of the vertebral bodies and the neural tube while stabilizing the trunk with the forceps. Take care not to damage the neural tube. Dissect the cartilage primordium by pulling the needle ventrally. Tear apart the tissue lateral to the neural tube to display the ventral part of the neural tube.
- Hold the neural tube with a Dumont #3 forceps and separate from dorsal muscle and epithelial tissue using another forceps.
- Collect the DRGs, which remain attached to the neural tube, using the tungsten needle. Pool the DRGs in ice-cold HBSS without phenol red.
- Centrifuge the DRGs for 2min at 2000rpm and withdraw the HBSS. Digest the DRGs by incubation in 0.25% trypsin and 3.5 mg collagenase type 1 in HBSS without Ca2+ and Mg2+ for 20min at 37°C.
- Stop the reaction by adding FBS to 10%, centrifuge the cells for 2min at 2000rpm, resuspend the cells in the appropriate medium, and plate the cells onto culture dishes that have been precoated with either fibronectin or pDL/fibronectin (see Section III,D,1).
b. Sciatic Nerve NCSCs.
- Isolate embryos as described earlier.
- Fix embryos on a wax support. Cut an opening dorsolateral to the hind limb, proximal to the spinal cord. Nerve and nerve plexus are revealed underneath muscle tissue.
- Fix the hind limb with a Dumont #5 forceps. With another #5 forceps, pull out sciatic nerve running into the hind limb. Dissect sciatic nerves into ice-cold HBSS without Ca2+ and Mg2+. Centrifuge cells at 2000 rpm for 2min, withdraw HBSS, and resuspend the pellet in a solution containing 0.025% trypsin and 1 mg/ml type 3 collagenase.
- Incubate for 4min at 37°C and then quench the digestion with 2 volumes of L15CO2 containing 1 mg/ml BSA, penicillin/streptomycin, and 25µg/ml deoxyribonuclease type 1.
- Centrifuge the cells at 2000rpm for 2min and slowly triturate them in medium.
l a. To prepare enteric NCSCs from embryos, dissect the entire gut distal to the stomach and digest in 1 mg/ml collagenase type I in HBSS without Ca2+ and Mg2+ for 20min at 37°C Preparations from older embryos are digested for 45 min in a solution that, in addition to the collagenase, contains 0.01% trypsin.
lb. Stop the digestion by adding FBS to 10%. Subsequently, centrifuge the cells for 2min at 1800rpm, triturate, and resuspend.
2a. To isolate and culture early postnatal gut NCSCs, separate the small intestine from the attached mesentry and place into ice-cold HBSS without Ca2+ and Mg2+. Peel free the outer muscle/plexus layers of the underlying epithelium, mince, and dissociate in 0.025% trypsin/EDTA plus 1 mg/ml type 4 collagenase in HBSS without Ca2+ and Mg2+ for 8 min at 37°C Quench the digestion with two volumes medium, centrifuge the cells, and triturate.
2b. Filter the cells through a nylon screen to remove clumps of cells and undigested tissue. Before plating, resuspend the cells in medium.
3. Flow Cytometry
Isolation of prospectively identified NCSCs by FACS avoids contamination by nonneural cells and allows enrichment of the NCSC population. Suspend dissociated cells in antibody-binding buffer, add the primary antibody (or mixture of antibodies) at the appropriate concentration, and incubate for 20-25 min on ice. Wash three times in antibody-binding buffer and incubate with fluorophore-conjugated secondary antibody. Wash cells and resuspend in buffer containing 2 µg/ml of the viability dye 7-aminoactinomycin D (7-AAD; Molecular Probes, Eugene, OR). This step allows exclusion of 7-AAD-positive dead cells during the FACS procedure. To isolate NCSCs from sciatic nerve, cells have been sorted that express the neurotrophin receptor p75 but not P0, a PNS myelin component (Morrison et al., 1999). For the isolation of gut NCSCs, a selection for p75/α4 integrin doublepositive cells has been performed (Bixby et al., 2002; Kruger et al., 2002). Prior to and after sorting, it is recommended to keep tissue culture plates in sealed plastic bags gassed with 5% CO2 to maintain the pH in the medium.
1. Substrate Preparation
Dishes coated with fibronectin: dilute 5 ml (one vial; 5 mg) fibronectin in 20ml sterile l× D-PBS. Apply 1 ml fibronectin to 35-mm Corning tissue culture dishes and withdraw it immediately and add the appropriate media. It is possible to reuse the fibronectin solution several times. Dishes coated with poly-D-lysine/ fibronectin: resuspend 5 mg poly-D-lysine in 10ml cell culture water. Rinse each 35 mm Corning tissue culture dish with 1 ml poly-D-lysine solution. Allow plates to air dry. Subsequently, wash twice with tissue culture water and air dry plates again. Apply fibronection to poly-D-lysine-coated plates as described earlier.
2. Culturing NCSCs from Neural Tube Explants in SM
In the absence of instructive growth factors (see Section III,D,5), the following conditions are permissive for the generation of autonomic neurons, peripheral glia, and nonneural smooth muscle-like cells. After neural tube isolation, the culture dishes are incubated at 37°C, 5% CO2 and 20% 02 (from air) for 24h (rat neural crest) to 48h (mouse neural crest) in SM. At this stage, most of the emigrated neural crest cells coexpress the transcription factor Soxl0 and the low-affinity neurotrophin receptor p75 as markers for undifferentiated NCSCs (Paratore et al., 2001; Stemple and Anderson, 1992). For further incubation, it is possible to scrape away the neural tube from the neural crest cells that migrated onto the substrate using an Lshaped tungsten needle and an inverted phasecontrast microscope equipped with a 5 or 10× objective lens (Fig. 1). Differentiated cell types become apparent after a few days of culture in SM (Stemple and Anderson, 1992). Differentiation is promoted by the addition of 10% FBS and 5 µM forskolin (Sommer et al., 1995).
In the presence of the neural tube and upon addition of NT3, BDNF, and LIE the generation of sensory neurons is observed proximal to the neural tube, in addition to the autonomic neurons that are found scattered throughout the outgrowth after 8 days in culture (Greenwood et al., 1999).
3. Culturing NCSCs from Neural Tube Explants in SN Medium
In the absence of instructive growth factors (see Section III, D,5), neural tube explants in SN medium consist of early NCSCs that can generate sensory neurons. After plating, neural tube explants are incubated in SN1 medium for 20 h. Outgrowth of NCSCs occurs during this period. To allow sensory neuronal differentiation, withdraw the SN1 medium from the plates after 48h of explant incubation and add SN2 medium for another 2 days.
Comments
Twenty hours after having plated the isolated neural tubes, the early neural crest explants cultured in SN1 express neither the sensory marker Brn-3A nor NF160, whereas virtually all neural crest cells express p75 and Soxl0 (Figs. 2A and 2B) (Hari et al., 2002). Sensory neurons obtained after prolonged incubation are characterized by coexpression of the POU domain transcription factor Brn-3A and NF160 (Fedtsova and Turner, 1995) (Figs. 2C and 2D).
Allow rat and mouse NCSCs to emigrate for 24 and 48 h, respectively. After scraping away the neural tubes, carefully wash plates once with DMEM. Detach the NCSCs by treatment with a 0.05% trypsin solution for 2min at 37°C Quickly resuspend the cells in DMEM+10% FBS to abolish the reaction. Centrifuge the cells at 2000 rpm for 2 min and resuspend the pellet in 1 ml fresh medium. Count the cells in a Neubauer counting chamber. Plate the cells at low density (100-300 cells/35-mm plate). Let the cells settle down for approximately 3 h. Single NCSCs are mapped by labeling the surface antigen p75 on living cells (Stemple and Anderson, 1992).
Staining is performed in SM for 30rain using a rabbit antimouse p75 antibody (1:300 dilution, Chemikon International). Wash the cells three times in DMEM and visualize the staining using a Cy3-coupled goat antirabbit IgG (Jackson Laboratories) in SM for 30 min at room temperature. Wash the cells three times in DMEM and add 1 ml fresh SM medium to each plate. Detect p75-expressing NCSCs with an inverted fluorescence microscope at 10× magnification. Mark single founder cells by inscribing them with a 3- to 4-mm circle using a grease pencil on the bottom of the dish.
![FIGURE 2 NCSCs are identified by coexpression of the transcription factor Soxl0 and the low-affinity neurotrophin receptor p75 (B). Neural tube explant cultures cultured in SN conditions for 20h were fixed with 3.7% formaldehyde in D-PBS for 10min. Cells were treated for 10min at room temperature with blocking buffer containing 10% goat serum, 0.3% Triton X-100, and 0.1% BSA in DPBS and were stained with rabbit antimouse p75 (1:300 dilution, Chemikon International) for 1 h at room temperature and with the monoclonal anti-Soxl0 antibody (1:10 dilution; Paratore et al., 2001) for 2h at room temperature. Within 4 days in culture, neural crest cells differentiate into sensory neurons identified by coexpression of the POU transcription factor Brn-3A and NF160 (D). Immunocytochemistry with the polyclonal rabbit anti-Brn-3A antibody [1:300 dilution (Fedtsova and Turner, 1995)] and monoclonal anti-NF160 antibody NN18 (1:300 dilution, IgG, Sigma-Aldrich) were carried out at room temperature for 1 h. Immunostainings were visualized by incubation for 1 h at room temperature using the following secondary antibodies at 1:200 dilution: Cy3-conjugated goat antimouse IgG, Cy3-conjugated goat antirabbit IgG, FITC-coupled donkey antirabbit IgG (Jackson Immuno Research Laboratories), and FITCcoupled horse antimouse IgG (Vector Laboratories). (A and C) Corresponding phase-contrast pictures.](images/v1_pa_s02_c08_f02.jpg) |
| FIGURE 2 NCSCs are identified by coexpression of the transcription factor Soxl0 and the low-affinity neurotrophin receptor p75 (B). Neural tube explant cultures cultured in SN conditions for 20h were fixed with 3.7% formaldehyde in D-PBS for 10min. Cells were treated for 10min at room temperature with blocking buffer containing 10% goat serum, 0.3% Triton X-100, and 0.1% BSA in DPBS and were stained with rabbit antimouse p75 (1:300 dilution, Chemikon International) for 1 h at room temperature and with the monoclonal anti-Soxl0 antibody (1:10 dilution; Paratore et al., 2001) for 2h at room temperature. Within 4 days in culture, neural crest cells differentiate into sensory neurons identified by coexpression of the POU transcription factor Brn-3A and NF160 (D). Immunocytochemistry with the polyclonal rabbit anti-Brn-3A antibody [1:300 dilution (Fedtsova and Turner, 1995)] and monoclonal anti-NF160 antibody NN18 (1:300 dilution, IgG, Sigma-Aldrich) were carried out at room temperature for 1 h. Immunostainings were visualized by incubation for 1 h at room temperature using the following secondary antibodies at 1:200 dilution: Cy3-conjugated goat antimouse IgG, Cy3-conjugated goat antirabbit IgG, FITC-coupled donkey antirabbit IgG (Jackson Immuno Research Laboratories), and FITCcoupled horse antimouse IgG (Vector Laboratories). (A and C) Corresponding phase-contrast pictures. |
For reasons not entirely clear, mouse NCSCs display a low survival capacity at clonal density. Clonal experiments with mouse NCSCs are therefore only possible under certain conditions (such as in the presence of fetal bovine serum) (Paratore et al., 2001). In the rat, clonogenic culture systems allowed assessment of the state of commitment of neural crest cells by exposing individual cells to changing environmental cues. Using such experiments, instructive growth factors have been identified that are able to promote the differentiation of NCSCs to specific lineages in vitro (Morrison et al., 1999, 2000b; Shah et al., 1994, 1996). Bone morphogenic protein 2 (BMP2) promotes a neuronal and, to a lesser extent, a smooth muscle-like fate, whereas single neural crest cells are instructed by transforming growth factor-β (TGFβ) to adopt a nonneural fate. Furthermore, individual neural crest cells choose a glial fate upon either Notch signal activation or treatment with GGF, an isoform of neuregulin 1 (NRG1). Finally, canonical Wnt signaling instructively promotes sensory neurogenesis in NCSCs (Lee et al., 2004). However, the response of NCSCs to instructive growth factors is modulated by short-range cell-cell interactions termed community effects and other signals (Hagedorn et al., 1999).
Moreover, serial subcloning experiments demonstrated the self-renewal capacity of NCSCs (Morrison et al., 1999; Stemple and Anderson, 1992). The signals promoting self-renewal and maintenance of NCSCs have not yet been discovered.
Cells are cultured either in 35-mm or in 6-well plates that have been precoated with poly-D-lysine and fibronectin (see earlier discussion). Both the traditional SM according to Stemple and Anderson (1992) and a simplified SM (Bixby et al., 2002; Morrison et al., 1999) have been used successfully to culture postmigratory NCSCs (Bixby et al., 2002; Hagedorn et al., 1999; Morrison et al., 1999). When using the simplified SM, incubate cells in SM1 for 6 days and then add SM2 for another 8 days to favor differentiation. For clonal analysis, directly plate cells at low density after dissociation of postmigratory neural crest target tissues (100-300 cells/35-mm plate; fewer than 30 cells per well of a 6-well plate).
Comments
Although neural crest cells isolated both from neural tube explant cultures and from various neural crest-derived tissues have been shown to be multipotent and responsive to instructive growth factors, cell-intrinsic differences between NCSCs from different origins affect fate decisions by changing the sensitivity of the cells to specific extracellular signals (Bixby et al., 2002; Kruger et al., 2002; Paratore et al., 2001; White et al., 2001).
6. Culturing NCSCs at Reduced Oxygen Levels
Reduced levels of oxygen have been shown to influence the survival, proliferation, and cell fate decision of neural stem cells (Morrison et al., 2000a). To culture neural tube explants at reduced oxygen levels, put all the dishes after neural tube isolation into a gas-tight modular incubator chamber and flush the chamber for 3-5 min with a custom gas mixture of 1% 02, 6% CO2, and balance N2 to generate an actual O2 level of 3-6%. The gas-tight chamber is housed inside a normal incubator. Once cultures are established in the reduced oxygen chamber, minimize the opening to avoid reperfusion.
We observed that in SN1 NCSCs appeared healthier after 20 h when cultured at reduced oxygen levels (M. K16ber et al., unpublished results). Moreover, culturing NCSCs at reduced oxygen levels in the presence of BMP-2 and forskolin revealed that low oxygen levels can influence cell fate (Morrison et al., 2000a).
IV. PITFALLS
- In order to establish NCSC cultures, follow the instructions carefully. Note that small differences in concentrations of media ingredients may influence outgrowth, proliferation, survival, and differentiation of NCSCs.
- Different batches of CEE and FBS might have different effects on the cultures. When using a new batch of CEE or FBS, always compare it to an older batch.
- During isolation, triturate the neural tubes slowly and patiently. Rapid trituration can damage the neural tubes, which can impair efficient neural crest outgrowth.
- Do not exceed the time of the digestion during isolation.
- Always use fresh media. Media should not be stored for more than 1 week.
The protocols described here have been developed over a number of years. They represent the work of many people, mainly from the laboratories of Dr. David J. Anderson and Dr. Sean J. Morrison and from our own laboratory. We thank Dr. Ned Mantei and Hye-Youn Lee for help with the manuscript and thank Drs. Andrew McMahon, Philippe Soriano, Eric Turner, and Michael Wegner for tools used to prepare the figures.
References
Anderson, D. J., Groves, A., Lo, L., Ma, Q., Rao, M., Shah, N. M., and Sommer, L. (1997). Cell lineage determination and the control of neuronal identity in the neural crest. Cold Spring Harb. Symp. Quant. Biol. 62, 493-504
Bixby, S., Kruger, G. M., Mosher, J. T., Joseph, N. M., and Morrison, S. J. (2002). Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron 35, 643-656.
Danielian, P. S., Muccino, D., Rowitch, D. H., Michael, S. K., and McMahon, A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326.
Fedtsova, N. G., and Turner, E. E. (1995). Brn-3.0 expression identifies early postmitotic CNS neurons and sensory neural precursors. Mech. Dev. 53, 291-304.
Hagedorn, L., Suter, U., and Sommer, L. (1999). P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-β family factors. Development 126, 3781-3794.
Hari, U, Brault, V., K16ber, M., Lee, H. Y., Ille, E, Leimeroth, R., Paratore, C., Suter, U., Kemler, R., and Sommer, L. (2002). Lineage-specific requirements of beta-catenin in neural crest development. J. Cell Biol. 159, 867-880.
Kruger, G. M., Mosher, J. T., Bixby, S., Joseph, N., Iwashita, T., and Morrison, S. J. (2002). Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35, 657-669.
Le Douarin, N. M., and Kalcheim, C. (1999). "The Neural Crest." Cambridge Univ. Press, UK.
Lee, H. Y., K16ber, M., Hari, L., Brault, V., Suter, U., Taketo, M. M., Kemler, R., and Sommer, L. (2004). Instructive Role of Wnt/β- Catenin in Sensory Fate Specification in Neural Crest Stem Cells. Science, 303, 1020-1023.
Morrison, S. J., Csete, M., Groves, A. K., Melega, W., Wold, B., and Anderson, D. J. (2000a). Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 20, 7370-7376.
Morrison, S. J., Perez, S. E., Qiao, Z., Verdi, J. M., Hicks, C., Weinmaster, G., and Anderson, D. J. (2000b). Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499-510.
Morrison, S. J., White, P. M., Zock, C., and Anderson, D. J. (1999). Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96, 737-749.
Paratore, C., Goerich, D. E., Suter, U., Wegner, M., and Sommer, L. (2001). Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Soxl0 and extrinsic combinatorial signaling. Development 128, 3949-3961.
Paratore, C., Hagedorn, L., Floris, J., Hari, L., K16ber, M., Suter, U., and Sommer, L. (2002). Cell-intrinsic and cell-extrinsic cues regulating lineage decisions in multipotent neural crest-derived progenitor cells. Int. J. Dev. Biol. 46, 193-200.
Pomeranz, H. D., Rothman, T. P., Chalazonitis, A., Tennyson, V. M., and Gershon, M. D. (1993). Neural crest-derived cells isolated from gut by immunoselection develop neuronal and glial phenotypes when cultured on laminin. Dev. Biol. 156, 341- 361.
Shah, N. M., Marchionni, M. A., Isaacs, I., Stroobant, P., and Anderson, D. J. (1994). Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell 77, 349-360.
Sommer, L. (2001). Context-dependent regulation of fate decisions in multipotent progenitor cells of the peripheral nervous system. Cell Tissue Res. 305, 211-216.
Sommer, L., Shah, N., Rao, M., and Anderson, D. J. (1995). The cellular function of MASH1 in autonomic neurogenesis. Neuron 15, 1245-1258.
Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70-71.
Stemple, D. L., and Anderson, D. J. (1992). Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 71, 973-985.
White, P. M., Morrison, S. J., Orimoto, K., Kubu, C. J., Verdi, J. M., and Anderson, D. J. (2001). Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron 29, 57-71.
Ziller, C., Dupin, E., Brazeau, P., Paulin, D., and Le Douarin, N. M. (1983). Early segregation of a neural precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell 32, 627-638.




