T-Cell Isolation and Propagation in vitro
Cellular immunity is largely based on Tlymphocytes. Like B cells, T cells also arise from the bone marrow. However, unlike B cells, they migrate to the thymus for maturation. A T-cell expresses a unique antigen-binding molecule called the T-cell receptor (TCR) on the cell surface. In contrast to membrane-bound antibodies on B cells, which can recognize the antigen alone, the majority of TCR recognizes a complex ligand, comprising an antigenic peptide bound to a protein called the major histocompatibility complex (MHC) [known in humans as human leukocyte antigen (HLA)] molecule (Moss et al., 1992). When a T-cell encounters an antigen in the context of an HLA molecule, it undergoes clonal expansion and differentiates into memory and various effector T-cells: CD4+ T-helper cells and CD8+ cytotoxic T-lymphocytes (CTL). Activation of both humoral and cell-mediated parts of the immune response requires cytokines produced by T-helper cells. The activation of T-helper cells is carefully regulated, and naive cells only become activated when they recognize an antigen presented by class II HLA molecules in context with the appropriate costimulatory molecules on the surface of professional antigen-presenting cells (macrophages, B cells, and dendritic cells) (Stockwin et al., 2000).
Until recently, measurements of the levels of cellular immune responses, i.e., those mediated by CD4+ and CD8+ T-lymphocytes, have depended largely on culture in vitro and the subsequent measurement of specific functions (such as cytolysis). More recently, new technologies based around tetrameric class I peptide complexes (tetramers) have allowed immunologists to isolate and measure CD8+ T-lymphocyte levels directly ex vivo. This article describes measures used to generate and clone specific T-cells in culture as well as measures to isolate specific T-cells by means of recombinant HLA/peptide complexes. Finally, it describes the conventional chrome release assay and the ELISPOT assay for the measurement of specific Tcell immunity. It should be noted, however, that T-cells behave very differently and that a T-cell protocol should not be considered as the definitive receipt but rather as a guideline that can be altered depending on the target or the donor. Finally, the making of dendritic cells has been described in detail elsewhere in this volume. However, this is an important first step of making primary T-cells and is consequently mentioned shortly here.
A. Tissue Culture Medium
X-vivo medium (Cambrex Bio Science, Cat. No. 04-418Q). Store at 4°C.
Human serum (Sigma, Cat. No. H1513). Store at-20°C.
Standard medium: X-vivo, 5% human serum. Store at 4°C.
RPMI 1640 medium (GIBCO, Cat. No. 61870-010). Store at 4°C.
Fetal calf serum (FCS) (Sigma, Cat. No. F7524). Store at 4°C.
R10 medium: RPMI 1640 + 10% FCS. Store at 4°C.
B. Cytokines
Interleukin (IL)-2 [Apodan, Cat. No. 004184]. Store at -80°C. Aliquot 20 units/µl.
IL-4 [Peprotech (trichem), Cat. No. 200-04]. Store at -20°C. Aliquot 10 units /µl.
IL-7 [Peprotech (trichem), Cat. No. 200-07]. Store at -20°C. Aliquot 5 ng/µl.
IL-12 [Peprotech (trichem), Cat. No. 200-12]. Store at -20°C. Aliquot 20 units/µl.
GM-CSF [Peprotech (trichem), Cat. No. 300-03]. Store at -20°C. Aliquot 800 units/µl.
TNF-α [Peprotech (trichem), Cat. No. 300-01A]. Store at-20°C. Aliquot 10ng/µl.
PHA (Sigma, Cat. No.). Store at-20°C. Aliquot 0.9mg/µl.
Anti-CD28 (eBioscience, Clone 28.8, Cat. No. 16-0288- 81). Store at 4°C.
Anti-CD3 (eBioscience, Clone OKT3, Cat. No. 14-0037- 82). Store at 4°C.
Tricolor-anti-CD8 (Caltag, Burlingame, CA, Cat. No.). Store at 4°C.
D. Sterile Plastic Plates
96-well plates [Boule (Corning Costar), Cat. No. 3799]
48-well plates [Boule (Corning Costar), Cat. No. 3548]
24-well plates [Boule (Corning Costar), Cat. No. 3526]
6-well plates [Boule (Corning Costar), Cat. No. 3516]
E. Additional Materials
Lymphoprep/Ficoll (Medinor, Cat. No. 30066.03). Store in the dark at 4°C.
51Crom (Amersham, Cat. No. CJS1) 5mCi/1ml. Store at-20°C. Dilute 1:5 in phosphate-buffered saline (PBS) before use.
Biotinylated monomers or PE-labeled tetramer (Proimmune, Oxford, UK). Store at 4°C.
Streptavin-coated magnetic beads (Dynabeads M-280, Dynal A/S, Cat. No. M-280). Store at 4°C.
Nitrocellulose plates (Millipore MAIPN 4550)
Coating antibody: Mab anti-hlFN γ clone 1-D1K, 1 mg/ml, MABTECH 3420-3. Store at 4°C. Dilute to 7.5µg/ml in PBS before use.
Secondary antibody: Biotinylated Mab anti-hlFN-γ, 7-b6-1, 1 mg/ml, MABTECH 3420-6. Store at 4°C. Dilute to 0.75µg/ml in diluting buffer before use.
Streptavidin AP: Calbiochem CAL 189732, 2ml. Add 2ml H20 and 2ml glycerol (85%). Store at 4°C. Dilute 1:1000 in diluting buffer before use.
NBT/BCIP substrate system. DAKO, Cat. No. K 0598. Store at 4°C. Dilute 1:5 in substrate buffer before use.
In addition to standard cell culture instruments:
Gamma counter (Cobra 5005, Packard Instruments, Meriden, CT)
ELISPOT counter [ImmunoSpot Series 2.0 analyzer (CTL Analyzers, LLC, Cleveland, OH]
Magnetic isolator (Dynal A/S, Oslo, Norway)
FACSVantage (Becton-Dickinson, Mountain View, CA)
A. Induction of Specific T Cells as Primary Responses
The following is a description on how to grow antigen-specific T-cells using peptide-loaded dendrictic cells as stimulator cells (Pawelec, 2000). This protocol can also be used to grow tumor-specific T cells if tumor lysate-loaded dendritic cells are used as stimulator cells as described.
1. Stimulator Cell (Dendritic Cell) Culture Day -7
Dilute 50ml blood 1:1 with RPMI medium and separate on lymphoprep by centrifugation for 30min at 1200rpm. Harvest mononuclear cells, mix with an equal volume of RPMI, and centrifuge at 1500rpm for 10min, followed by two washes in R10 (1200rpm, 5 min). Resuspend cells to 20 × 106 cells/ml and plate out at 3 ml/well in 6-well plates. Incubate the cells for 2h at 37°C. Remove nonadherent cells (lymphocytes) by gentle suction. If necessary, the lymphocytes may be frozen. Add 2.5 ml standard medium containing 800 units/ml GM-CSF and 500 units/ml IL-4 to each well.
Day -5
Add 2.5ml standard medium containing 1600 units / ml GM-CSF and 1000 units / ml IL-4.
Day -3
Remove 2.5ml of medium from each well and replace with 2.5ml of fresh medium containing 1600 units / ml GM-CSF and 1000 units / ml IL-4.
Day -1
Remove 1 ml of medium from each well and replace with 1 ml of fresh medium containing 10ng/ml of TNF-α.
Harvest the cultured DC and wash twice in RPMI medium. Resuspend in 1 ml RPMI medium containing 50µg/ml peptide and 3µg/ml β2m. Incubate the cells for 4h at 37°C; gently resuspend every hour. Irradiate at 25 Gy, wash twice with RPMI medum, and resupend cells (stimulator cells) at 3 × 105/ml in standard medum.
2. Initiation of Primary T-Cell Culture
Mix 3 × 106 freshly isolated lymphocytes and 3 × 105 peptide-pulsed stimulator cells in a 24-well plate at 2ml/well.
Day 1
Add IL-7 to a final concentration of 5ng/ml and 100 pg/ml IL-12.
Day 7
Remove 1 ml of medium and replace with 1 ml of standard medium containing 20ng/ml of IL-7.
Day 12
Harvest responder cells, separate over Ficoll, wash once, and count viable cells. Resuspend at 1.5 × 106/ml in standard medium and keep tube at 37°C. Thaw 2 × 106 autologous peripheral blood mononuclear cells (PBMC) per 1.5 × 106 responder cells. Wash the PBMC once in RPMI medium and irradiate at 60Gy. Wash again in RPMI and incubate 2h at 37°C with 20µ g/ml peptide and 2µ/ml β2m. Remove medium and gently wash once in RPMI. Mix 1.5 × 106/ml and 2 × 106 peptide-pulsed autologous PBMC in 2ml of standard medium. Alternatively, instead of peptidepulsed PBMC, use peptide-pulsed DC prepared as on Day 0.
Day 14
Remove 1 ml of medium and replace with 1 ml of fresh medium containing 40 units/ml IL-2 to each well.
Day 19
Restimulate as on day 12. Add IL-2 to the culture as on day 14. Restimulation is needed four or five times before a primary response is measurable.
B. Cloning of T Cells by Limiting Dilution
Day 0
Wash autologous PBMC once in RPMI medium and irradiate at 30Gy. Wash again in RPMI and incubate 2h at 37°C with 20µg/ml peptide and 2µg/ml β2m. Plate T-cells at limiting dilution (featuring 10; 3; 1; 0.3 cells/well) in 96-well round-bottom microtiter plates containing 105 irradiated, autologous peptidepulsed PBL, 10 units/ml IL-4, and 40 units/ml IL-2 in 100 µl standard medium.
Day 4
Add 50µl standard medium containing IL-2 and IL-4 to a final concentration of 10 and 40 units/ml, respectively.
Day 8
Add 50µl standard medium containing IL-2 and IL-4 to a final concentration of 10 and 40 units/ml, respectively.
Inspect cells for growing cells microscopically. Transfer growing cells to 48-well plates containing peptide-pulsed autologous feeder cells and antigen. At the same time, test clones for antigen specificity by the chrome release assay or ELISPOT. Incubate plates 7 days at 37°C in a 5% CO2 incubator adding IL-2 and IL-4 every third day and transfer antigen-specific T-cells to 24-well plates.
An Alternative Cloning Protocol Using Anti-CD3 and Anti-CD28 Antibodies
This procedure is modified from Oelke et al. (2000).
Day -1
Coat a 96-well plate with 100ng/ml of anti-CD3 and anti-CD28 antibodies in PBS for 24h at room temperature.
Day 0
Plate T cells at limiting dilution in precoated 96-well plates containing 105 irradiated, autologous PBL, 10 units/ml IL-4, and 40 units/ml IL-2 in 100 µl standard medium. Continue as described in previously.
C. Expansion of T-Cell Clones
This is a protocol for the expansion of already established clones modified from Dunbar et al. (1998). The cloning mix described in the following can, however, also be used as stimulators to clone T-cells instead of autologous PBL as described previously.
Day 1
For preparing the cloning mix isolate fresh lymphocytes from at least three individuals, resuspend them in standard medium, and irradiate (20Gy). Count the lymphocytes and mix them together to give a final total concentration of 1 × 106/ml. Add PHA to a final concentration of lµg/ml and leave in the incubator while the clone is thawed and counted. Thaw the clone, count, and resuspend in prewarmed cloning mix at between 105 and 5 × 105 cellsml. Plate out into a U-bottomed, 96-well plate at 100µl/well.
Day 3
Add 100ml medium and 40 units/ml IL-2 to each well.
Prepare fresh cloning mix, plate 1 ml per well into 24-well plates and prewarm in an incubator.
Transfer I well of the 96-well plate into I well of the prewarmed 24-well plate.
Day 10
Add 1 ml of medium and 40 units/ml IL-2 to each well.
Day 14
Pool the wells for each individual clone, count, and remove the required amount of cells for a chrome release assay to check if the clones have maintained antigen specificity. Freeze the remaining cells in aliquots of 2 to 7 million per vial.
D. Isolation of Peptide-Specific T Cells
Momomeric or tetrameric MHC/peptide complexes can be bought commercially, e.g., proimmune (Oxford, UK). They can, however, also be made as described by Pedersen et al. (2001). However, these rather complex procedures are not the scope of this review.
1. Isolation of Specific T-Cells Using MHC Class I/Peptide Complexes Coupled to Magnetic Beads
This procedure is performed according to Andersen et al. (2001; Schrama et al., 2001). Incubate 2.5µg biotinylated monomer of a given HLA/peptide complex with 5 × 106 streptavin-coated magnetic beads in 40µl PBS for 20min at room temperature. Wash the magnetic complexes three times in PBS using a magnetic field.
Add 107 PBL or lymphocytes in 100µl PBS with 5% bovine serum albumin (BSA) and rotate very gently for I h. Wash the antigen-specific T-cells associating with the magnetic complexes gently three times in PBL. Incubate for 2 h at 37°C and resuspend several times in standard medium to release the cells from the magnetic beads.
Assay the antigen specificity of isolated antigenspecific CD8+ T-cells in an ELISPOT or chrome release assay after at least 5 days in culture.
2. Isolation of Specific T-Cells by FACS
This protocol is modified from Dunbar et al. (1999). Culture PBL overnight in standard medium before sorting. Alternatively, pulse PBL with 10 µM peptide in standard medium, plus 10U/ml IL-7 and culture for 10 days before sorting. Stain cells with PE-labeled tetrameric HLA/peptide complex for 15min at 37°C before the addition of tricolor-anti-CD8 for 15min on ice. Wash the cells six times in PBS before analysis on FACS.
E. Examination of Antigen Specificity
1. Crome Release Assay
Conventional 51Cr release assays for CTL-mediated cytotoxicity can be used to test the specificity of CTL lines against relevant target cells, e.g., autologous EBVtransformed B-cell lines or cancer cell lines. This procedure is performed according to Brunner et al. (1968).
Label 106 target cells in 50 µlR10 mediumd with [51Cr] (100µCi) in a round-bottomed well of a 96-well plate at 37°C for 60min. If necessary, add 4µg of peptide. Wash the target cells four times and plate out in 96- well plates in 100µl R10 medium. Add T-cells at various effector:target ratios in another 100µl R10 and incubate at 37°C for 4 h. Aspirate 100 µl of medium and count [51Cr] release in a gamma counter. Determine the maximum [51Cr] release in separate wells by the addition of 100µl 10% Triton X-100 and the spontaneous release by the addition of 100µl R10 only to target cells.
Calculate the specific lysis using the following formula:
[(experimental release - spontaneous release)/ (maximium release- spoontaneous release)] × 100.
2. ELISPOT
For the measurement of specific T-cell immunity, ELISPOT analysis, involving the incubation of primary PBMC with one or more peptide epitopes, is probably the most sensitive and reliable assay (Pittet et al., 1999). ELISPOT is based on the detection of the peptideinduced release of cytokines such as interferon (IFN)- γ by single T-cells upon stimulation with a peptide antigen (Scheibenbogen et al., 1997).
Solutions
PBS
Washing buffer: PBS and 0.05% Tween 20; store at room temperature
Diluting buffer: PBS, 1% BSA, and 0.02% NaN3; store at room temperature
Substrate buffer: 0.1M NaCl, 50mM MgCl2, and 0.1M Tris-HCl, pH 9.50; store at room temperature
Coating of Plates
Add 75µl 7.5 ug/ml coating antibody. The antibody concentration may change depending on the cytokine target. Leave overnight at room temperature (if coating 3-5 days in advance, leave at 4°C). Wash the plate with 6 × 200 µl PBS. Block the plate with 200 µl media. Leave in incubator for 2h.
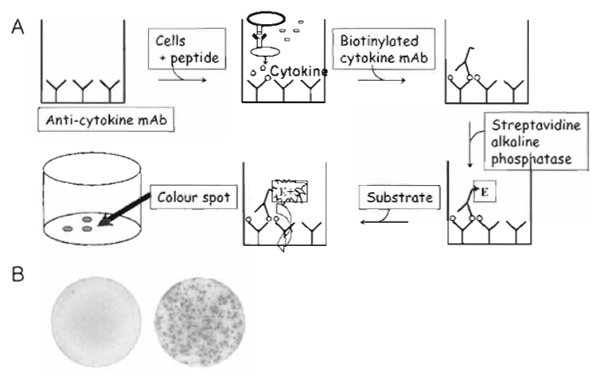 |
| FIGURE 1 (A) Schematic illustration of the ELISPOT assay. Cytokine-specific antibodies are coated onto nitrocellulose filter plates to capture the secreted cytokine; a peptide-pulsed target cell is added together with cells containing the precursor T-cells. If a T-cell recognizes the peptide epitope examined, the cell releases cytokine. This can be detected as a spot by a colorimetric reaction with secondary antibodies. Thus, the spot represents the cytokine after secretion by a single activated cell. (B) ELISPOT wells after incubation with T-cells that were either nonreactive (left) or reactive (right) against the antigen examined. |
Setting up the ELISPOT
Prepare the serial dilutions of the cells (sterile) at relevant concentrations in order to add the cells to each well in 200 µl media. Poor off the blocking media, and add 200 µl cells and 0.5 µl peptide (2mM) to each well.
Add 75 µl streptavidin (diluted 1:1000) to each well. Incubate for 1 h at room temperature. Wash the plate with 6 × 200 µl washing buffer and 1 × 200µl substrate buffer. Mix the substrate: 10ml substrate buffer + 44 µl NBT + 33 µl BCIP. Add 75 µl fresh substrate to each well. Leave at room temperature for 2-20min. Stop the reaction with tap water when spot development is satisfactory.
Count the spots using the ImmunoSpot Series 2.0 analyzer and calculate the peptide-specific T-cell frequency from the number of spot-forming cells.
IV. COMMENTS AND PITFALLS
It is always optimal to prepare T-cell cultures from fresh material. T-cells behave very differently, and it is always important to carefully inspect T-cell cultures daily in a microscope. Thus, it is always possible to alter the T-cell protocol depending on the target or the donor, e.g., the concentration of cytokines, the addition of new cytokines, the amount of antigen-presenting cells, the day of restimulation, and the addition of antibodies such as anti-CD28 anti-CD3, or other costimulatory factors. Additionally, T-cells should never be kept at too low cell densities, as cell-to-cell contact is very important. This is also why round-bottom wells are used instead of flat-bottom wells during the cloning of T-cells. Furthermore, when T-cells are transferred from small to larger wells, it is important to carefully inspect the cells microscopically to ensure that the cells are in good condition. In this regard, feeder cells (e.g., irradiated, autologous PBL) may be added if needed.
Andersen, M. H., Pedersen, L. O., Capeller, B., Brocker, E. B., Becker, J. C., and thor, S. R (2001). Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 61, 5964-5968.
Brunner, K. T., Mauel, J., Cerottini, J. C., and Chapuis, B. (1968). Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology 14, 181-196.
Castelli, C., Rivoltini, L., Andreola, G., Carrabba, M., Renkvist, N., and Parmiani, G. (2000). T-cell recognition of melanomaassociated antigens. J. Cell Physiol. 182, 323-331.
Dunbar, P. R., Chen, J. L., Chao, D., Rust, N., Teisserenc, H., Ogg, G. S., Romero, P., Weynants, P., and Cerundolo, V. (1999). Cutting edge: Rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J. Immunol. 162, 6959-6962.
Dunbar, P. R., Ogg, G. S., Chen, J., Rust, N., van der Bruggen, P., and Cerundolo, V. (1998). Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T-lymphocytes from peripheral blood. Curr. Biol. 8, 413-416.
Fu, Y. X., and Chaplin, D. D. (1999). Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17, 399- 433.
Moss, P. A., Rosenberg, W. M., and Bell, J. I. (1992). The human T-cell receptor in health and disease. Annu. Rev. Immunol. 10, 71-96.
Pawelec, G. (2000). New methods to approach immunotherapy of cancer-and strategies of tumours to avoid elimination. Conference report, on behalf of EUCAPS. European Cancer Research Consortium. Cancer Immunol. Immunother. 49, 276-280.
Pedersen, L. O., Nissen, M. H., Nielsen, L. L., Lauemoller, S. L., Hansen, N. J. V., Blicher, T., Hansen, A., Hviid, C. S., Thomsen, A. R., and Buus, S. (2001). Efficient assembly of recombinant major histocompatibility complex class I molecules with preformed disulfide bonds. Eur. J. Immunol. 31, 2986-2996.
Pittet, M. J., Valmori, D., Dunbar, P. R., Speiser, D. E., Lienard, D., Lejeune, E, Fleischhauer, K., Cerundolo, V., Cerottini, J. C., and Romero, P. (1999). High frequencies of naive Melan-A/MART-1- specific CD8(+) T-cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 190, 705-715.
Scheibenbogen, C., Lee, K. H., Mayer, S., Stevanovic, S., Moebius, U., Herr, W., Rammensee, H. G., and Keilholz, U. (1997). A sensitive ELISPOT assay for detection of CD8+ T-lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin. Cancer Res. 3, 221-226.
Schrama, D., Andersen, M. H., Terheyden, P., Schroder, L., Pedersen, L. O., thor Straten, P., and Becker, J. C. (2001). Oligoclonal T-cell receptor usage of melanocyte differentiation antigen-reactive T-Cells in stage IV melanoma patients. Cancer Res. 61, 493-496.
Stockwin, L. H., McGonagle, D., Martin, I. G., and Blair, G. E. (2000). Dendritic cells: Immunological sentinels with a central role in health and disease. Immunol. Cell Biol. 78, 91-102.




