Aspartate Aminotransferase
Many enzymes employ exogenous molecules known as
cofactors to assist in executing their chemistry. Sometimes
these cofactors are covalently bound to the enzyme and
sometimes not. Many types of cofactors are known, and
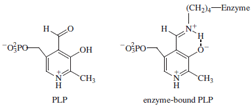
here we will focus on a well-studied example called pyridoxal
phosphate (PLP), which often participates in the
metabolism of amino acids. PLP, derived from vitamin
B6, is a covalently bound cofactor; it is attached to lysine
residues by means of a Schiff base or imine linkage as
shown at right.
The substrates for most PLP-requiring processes are
α-amino acids, and most of the processes take place at
the α-carbon position, although some take place at the
β- or γ -carbon. The enzymes which use PLP catalyze a
wide range of reactions, including racemizations, decarboxylations,
and amine transfers. In general, for all three
of these classes of reactions at the α-carbon the substrate
displaces the lysine and forms an aldimine intermediate
with the PLP.
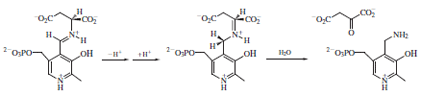
The now very acidic α-proton of the amino acid is
abstracted by a basic amino acid residue (often the
displaced lysine), with the pyridine ring of PLP acting
as an electron sink. For the racemases, a proton is
then delivered to the opposite face from the same or a
different basic residue with the net result of inversion
of configuration at the α-carbon. Attack of the active
site lysine effects product release and regenerates the
cofactor.
The structure of one PLP-utilizing transaminase,
aspartate aminotransferase, is shown in Fig. 8. This
enzyme catalyzes the reversible transamination reaction
shown below.
![Structure of an aspartate aminotransferase. The protein is a homodimer, with one covalently bound pyridoxal phosphate (shown in black) in each of the two subunits. The expanded view shows the cofactor in greater detail. [Adapted from Rhee, S. et al. (1997). “Refinement and comparisons of the crystal structures of pig cytosolic aspartate aminotransferase and its complex with 2-methylaspartate,” J. Biol. Chem. 272, 17293–17302.]](images/2-8.gif) |
| Figure 8 Structure of an aspartate aminotransferase. The protein is a homodimer, with one covalently bound
pyridoxal phosphate (shown in black) in each of the two subunits. The expanded view shows the cofactor in greater
detail. [Adapted from Rhee, S. et al. (1997). “Refinement and comparisons of the crystal structures of pig cytosolic
aspartate aminotransferase and its complex with 2-methylaspartate,” J. Biol. Chem. 272, 17293–17302.] |
In the transamination reaction, formation of the aldimine
intermediate between aspartate and PLP and its
deprotonation proceeds as described above for the racemases.
However, reprotonation occurs not at the same
carbon as in the racemization mechanism but at a position
adjacent to the PLP heterocycle.
Hydrolysis releases the product oxaloacetate and generates
a new form of the cofactor called pyridoxamine. The
reverse reaction is then carried out on the other substrate,
α-ketoglutarate, forming glutamate and regenerating the
PLP cofactor.
Reactions at the β-position (for example, in threonine
dehydatase) or the γ -position (in methionine-γ -lyase)
also proceed by means of formation of an aldimine intermediate
with the α-carbon of an α-amino acid. Such a survey
of PLP-dependent enzymes illustrates the important
point that one cofactor can be used for different kinds of
transformations. The reactions described all go through a
common aldimine intermediate, with the ultimate course
of the reaction being controlled by the appropriate substrate
specificity and positioning of amino acid side chains.
This flexibility allows nature to expand its chemical repertoire
with a relatively small set of cofactors.
There are other organic cofactors such as thiamine
pyrophosphate and biotin that participate in carbon–
carbon bond formation and cleavage, cofactors that
participate in reduction/oxidation, or redox, reactions
such as nicotinamide and flavin moieties discussed in
some of the earlier examples, and still others that are
metal based such as vitamin B
12 and porphyrin, which is
our next topic.
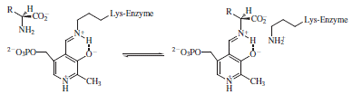
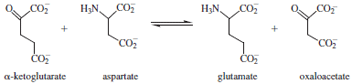
![Structure of an aspartate aminotransferase. The protein is a homodimer, with one covalently bound pyridoxal phosphate (shown in black) in each of the two subunits. The expanded view shows the cofactor in greater detail. [Adapted from Rhee, S. et al. (1997). “Refinement and comparisons of the crystal structures of pig cytosolic aspartate aminotransferase and its complex with 2-methylaspartate,” J. Biol. Chem. 272, 17293–17302.]](images/2-8.gif)