Carotenoids of Food Colors
Many of the yellow, orange, and red colors of plants and animals are due to carotenoids, pigments similar to those of carrots. The basic structure of carotenoids is a chain of eight isoprenoid units. Certain isoprenoid derivatives with shorter chains (e.g., vitamin A) are also considered carotenoids. Most of the structural differences among carotenoids exist at the ends of the chain. Some carotenoids are hydrocarbons and are known as carotenes, while others contain oxygen and are called xanthophylls. The structures of several carotenoids, along with the foods or tissues in which they are present, are shown in Table I.Because of the numerous double bonds in the carotenoid molecule, a large number of cistrans isomers are theoretically possible. The carotenoids of foods, however, are usually in the all-trans form (Table I). Trans to cis transformation is possible and is accelerated by heat, light, and acidity.
Carotenoids occur free or as esters of fatty acids or as complexes with proteins and carbohydrates; for example, in paprika, capsanthin is esterified with lauric acid. In live lobster, astaxanthin is complexed with protein; the astaxanthin–protein complex is blue-gray, the color of live lobster, but on heating, the complex is broken and the freed astaxanthin imparts its red color to the cooked lobster.
Carotenoids are present in a large variety of foods, from yeast and mushrooms, to fruits and vegetables, to eggs, to fats and oils, to fish and shellfish. As fat-soluble substances, carotenoids tend to concentrate in tissues or products rich in lipids, such as egg yolk and skin fat, vegetable oils, and fish oils.
TABLE I Types of Carotenoids and Their Natural Sources
| Structure | Name and source |
|---|---|
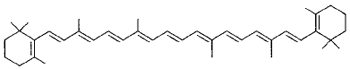 |
β-Carotene (carrot, egg, orange, chicken fat) |
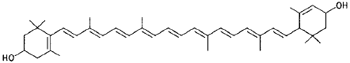 |
Xanthophyll (vegetables, egg, chicken fat) |
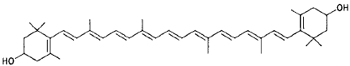 |
Zeaxanthin (yellow corn, egg, liver) |
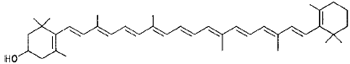 |
Cryptoxanthin (egg, yellow corn, orange) |
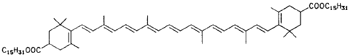 |
Physalien (asparagus, berries) |
| Bixin (annatto seeds) | |
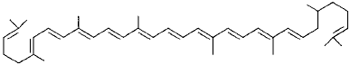 |
Lycopene (tomato, pink grapefuit, palm oil) |
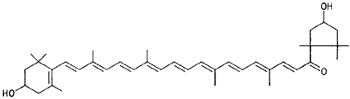 |
Capsanthin (paprika) |
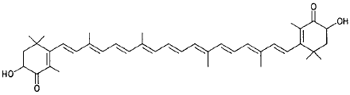 |
Astaxanthin (lobster, shrimp, salmon) |
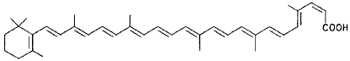 |
Torularhodin (Rhodotorula yeast) |
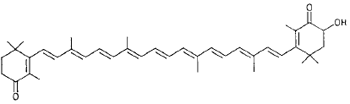 |
Canthaxanthin (mushrooms) |
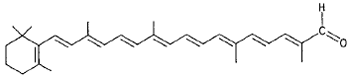 |
β-Apo-8´-carotenal (spinach, orange) |
 |
The stability of carotenoids in foods varies greatly, from severe loss to actual gain in carotenoid content during storage. Carotenoid losses amounting to 20 or 30% have been observed in dehydrated vegetables (e.g., carrots, sweet potatoes) stored in air. These losses are minimized when the dry product is stored in vacuum or inert gas (e.g., nitrogen), at low temperatures, and protected from light. The main degradative reaction of carotenoids is oxidation. Oxygen may act either directly on the double bonds or through the hydroperoxides formed during lipid autoxidation. Hydroperoxides formed during enzymatic lipid oxidation can also bleach carotenoids by a coupled lipid–carotenoid oxidation mechanism. On the other hand, certain vegetables, such as squash and sweet potatoes, in which carotenoid biosynthesis continues after harvesting, may manifest an increase in carotenoid content during storage.




