Nonenzymatic Browning
A number of chemical processes not involving enzymes may result in food browning. Briefly discussed here are the Maillard reaction, caramelization, ascorbic acid browning, and metalpolyphenol browning.1. The Maillard Reaction (Maillard Browning)
This reaction is actually a series of reactions occurring from the first encounter of a carbonyl compound with an amine compound to the formation of brown pigments. It is also known as the carbonyl–amine reaction, and its brown products are often called melanoidins, indicating their visual similarity to the melanins of enzymatic browning. The most common carbonyl compounds of foods involved in the Maillard reaction are reducing sugars, and the most common amine compounds are amino acids.
The intermediate reactions and their relative velocities vary with the type of initial reactants and the conditions of the reactions. Among sugars, pentoses are more reactive than hexoses, and hexoses are more reactive than reducing disaccharides. When free amino acids react with sugars, lysine appears to be the most active among them. In peptides and proteins, the N-terminal amino acid is the most reactive, followed by a nonterminal lysine. Raising the temperature and/or the pH accelerates the Maillard reaction. Intermediate water activity appears to maximize this reaction.
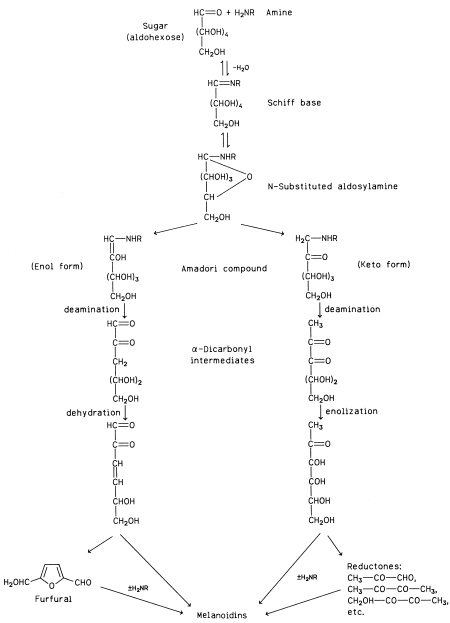 |
| Figure 14 Simplified scheme of melanoidin formation by the sugar–amine reaction (± means presence or absence). |
Several pathways have been proposed for the formation of melanoidins through the interaction of carbonyl and amine compounds. A simplified scheme is shown in Fig. 14. This scheme involves first the condensation of a carbonyl compound (an aldohexose in this scheme) with an amine to a Schiff base via an intermediate product (not shown). The Schiff base quickly cyclizes to an Nsubstituted glycosylamine. Up to this step the process is reversible because the glycosylamine can be hydrolyzed back to the initial reactants. The N-substituted glycosylamine is then rearranged to an N-substituted 1-amino- 1-deoxy-2-ketose, the Amadori compound, which is in equilibrium with its enol form. If the initial carbonyl compound is a 2-ketose (e.g., fructose), the corresponding Nsubstituted 2-amino-2-deoxy-1-aldose is formed by the Heyns rearrangement, which is analogous to the Amadori rearrangement for aldoses. The Amadori and Heyns compounds are subsequently subjected to a variety of transformations, which may include deamination, dehydration, enolization, cyclization, and degradation. The products of those reactions finally condense or polymerize with or without the participation of an additional amino compound to form dark pigments of a colloidal nature and ill-defined composition—the melanoidins. Some of the intermediate products are collectively called reductones, because they are strongly reducing compounds that account for the reducing properties of systems undergoing Maillard browning.
It should be mentioned here that a side reaction of the Maillard browning results in the formation of flavorful compounds, such as those associated with the roasting of meat, coffee, or nuts and the baking of bread. This side reaction, known as the Strecker degradation, occurs between α-amino acids and dicarbonyl compounds and leads to the formation of aldehydes possessing one less carbon atom than the corresponding initial amino acids. The newly formed aldehydes are responsible for the pleasing flavors.
A significant consequence of the Maillard reaction is the loss of the nutritional value of the amino acid involved in the reaction. If the participating amino acid is essential, and especially if it is a limiting one, as is lysine in most cereal grains, the Maillard reaction can seriously lower the nutritive value of the food. Toasting, for example, may reduce to one-half the protein efficiency ratio of bread.
2. Caramelization
This reaction leads to brown products when sugars are heated dry or in solution. Certain conditions of caramelization favor the formation of flavor compounds as well. The chemical transformations involved in caramelization are complex and poorly understood. They include dehydration, fragmentation, and polymerization. On the heating of pentoses, furfural is formed which polymerizes to brown products. Heating hexoses results in hydroxymethylfurfural, which polymerizes similarly. The large quantities of industrial caramel color that are added to beverages (cola drinks), baked goods, and confections are made by heating high-conversion corn syrups in the presence of catalysts (acids, alkalis, salts).
3. Ascorbic Acid Browning
When ascorbic acid is heated in the presence of acids, furfural is formed. The latter, either by itself or after reacting with amino compounds, polymerizes to brown products. Citrus juices, especially their concentrates, develop browning, which has been attributed to ascorbic acid degradation.
4. Metal–Polyphenol Browning
Polyphenolic compounds form complexes with certain metals. The polyphenols of fruits and vegetables most commonly chelate iron. The resulting iron complexes are bluish black pigments. Cutting apples with a nonstainless- steel knife results in darkening of both the blade and the cut surface of the apple. This darkening is independent of the enzymatic browning that might develop as a result of cutting.Wine makers avoid contact between the wine and iron implements because of the black iron–tannin precipitate that forms on such contact. Certain varieties of potatoes tend more than others to darken after cooking. This darkening is attributed to a complex between iron and chlorogenic acid. The iron of the tissue must first be oxidized to the ferric state for the blackish complex to appear. The stem end, which contains much less citric acid than the rest of the tuber, displays the deepest darkening. Canned or pickled cauliflower may turn dark due to the interaction of polyphenols in the tissue with iron from external sources.
As already indicated, nonenzymatic browning is desirable in certain instances and undesirable in others. The availability of reactants and the type of conditions (temperature, pH, moisture) will determine the extent of browning. A chemical preservative often used to inhibit nonenzymatic (and enzymatic) browning is sulfur dioxide. An obvious way to prevent metal-polyphenol browning is to eliminate contact between susceptible tissues and reactive metals and use inoffensive equipment (stainless steel, glass-lined tanks, etc.)




