Heme Pigments
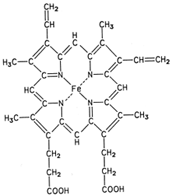 |
| Figure 1 Structure of heme. |
Myoglobin is a protein that facilitates the transfer of oxygen in muscles. It was the first protein to be fully elucidated with regard to the three-dimensional arrangement of its atoms. Hemoglobin, the oxygen-carrying pigment of blood, is composed of four heme groups attached to four polypeptide chains.
The myoglobin in meat is subject to chemical and color changes. Freshly cut meat looks purplish. On exposure to air, the surface of the meat acquires a more pleasing red hue (blooming of the cut). The color change is due to the oxygenation of myoglobin (an oxygen molecule is attached to the heme group in a fashion parallel to the oxygenation of hemoglobin). The oxygenated myoglobin is called oxymyoglobin. When meat is packed in plastic film, the oxygen permeability of the film should be sufficient to keep the myoglobin oxygenated. In both myoglobin and oxymyoglobin the heme iron is in the Fe2+ form. In the presence of oxygen, myoglobin is eventually oxidized to brown metmyoglobin, in which the heme iron is in the Fe3+ form. Both the oxygenation and oxidation processes are reversible. Severe oxidative deterioration may result in the formation of green pigments (sulfmyoglobin, cholemyoglobin).
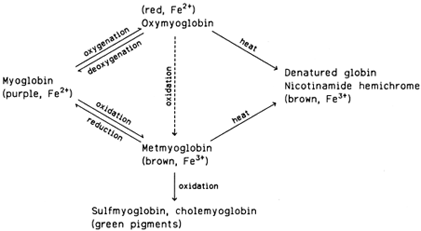 |
| Figure 2 Pigment changes in fresh and heated red meat. |
In cured meats, in which nitrite is used, many reactions occur, some of which lead to color changes. Among the established reactions are the following:
- the nitrite salt is converted to nitric oxide (NO), nitrate, and water;
- the NO replaces the H2O attached to the iron of heme and forms nitrosyl myoglobin, which is reddish;
- on heating, the nitrosyl myoglobin is transformed to nitrosyl hemochrome, which has the familiar pink color of cured meats; and
- any metmyoglobin present in the cured meat is similarly nitrosylated, reduced, and finally converted to nitrosyl hemochrome.




