Class Insecta
Class Insecta
Insecta (L. insectus, cut into) are the most diverse and abundant of all groups of arthropods. There are more species of insects than species of all other classes of animals combined. The number of insect species has been estimated at up to 10 million. There is also striking evidence of continuing evolution among insects at the present time, even though the fossil record indicates that the group as a whole is stable.
It is difficult to appreciate fully the significance of this extensive group and its role in the biological pattern of animal life. The study of insects (entomology) occupies the time and resources of skilled men and women all over the world. The struggle between humans and their insect competitors seems to be endless, yet paradoxically insects have so interwoven themselves into the economy of nature in so many useful roles that we would have a difficult time without them.
Insects differ from other arthropods in having three pairs of legs and usually two pairs of wings on the thoracic region of the body, although some have one pair of wings or none. Insects range in size from less than 1 mm to 20 cm in length, the majority being less than 2.5 cm long. Generally, the largest insects live in tropical areas.
Distribution
Insects are among the most abundant and widespread of all land animals. They have spread into practically all habitats that will support life except most of the sea. Relatively few are marine. Marine water striders (Halobates), which live on the surface of the ocean, are the only marine invertebrates that live on the sea-air interface. Insects are common in brackish water, in salt marshes, and on sandy beaches. They are abundant in fresh water, in soil, in forests (especially the tropical forest canopy), and in plants, and they are found even in deserts and wastelands, on mountaintops, and as parasites in and on plants and animals.
Their wide distribution is made possible by their powers of flight and their highly adaptable nature. In most cases they can easily surmount barriers that are virtually impassable to many other animals. Their small size allows them to be carried by currents of both wind and water to far regions. Their well-protected eggs can withstand rigorous conditions and can be carried long distances by birds and other animals. Their agility and aggressiveness enable them to fight for every possible niche in a habitat. No single pattern of biological adaptation can be applied to them.
Adaptability
Insects, during their evolution, have shown an amazing adaptability, as evidenced by their wide distribution and enormous diversity of species. Most of their structural modifications have taken place in wings, legs, antennae, mouthparts, and alimentary canal. Such wide diversity enables this vigorous group to take advantage of all available food and shelter. Some are parasitic, some suck the sap of plants, some chew the foliage of plants, some are predaceous, and some live on the blood of various animals. Within these different groups, specialization occurs, so that a particular kind of insect will eat, for instance, leaves of only one kind of plant. This specificity of eating habits lessens competition with other species and to a great extent accounts for their biological diversity.
Insects are well adapted to dry and desert regions. The hard and protective exoskeleton helps prevent evaporation. Some insects also extract most of the water from food, fecal material, and by-products of cell metabolism.
As in other arthropods, the exoskeleton is made up of a complex system of plates known as sclerites, connected by concealed, flexible hinge joints. Muscles between sclerites enable insects to make precise movements. Rigidity of their exoskeleton is attributable to the unique scleroproteins and not to its chitin component. Its lightness makes flying possible. By contrast, the cuticle of crustaceans is stiffened mostly by minerals.
External Form and Function
Insects show a remarkable variety of morphological characteristics, but they are much more homogeneous in tagmatization than are Crustacea. Some insects are fairly generalized in body structure; some are highly specialized. Grasshoppers, or locusts, are a generalized type often used in laboratories to demonstrate the general features of insects (Figure 20-4).
Insect tagmata are head, thorax, and abdomen. The cuticle of each body somite typically is composed of four plates (sclerites), a dorsal notum (tergum), a ventral sternum, and a pair of lateral pleura. Pleura of abdominal segments are membranous rather than sclerotized.
The head usually bears a pair of relatively large compound eyes, a pair of antennae, and usually three ocelli. Antennae, which vary greatly in size and form (Figure 20-5), act as tactile organs, olfactory organs, and in some cases as auditory organs. Mouthparts, formed from specially hardened cuticle, typically consist of a labrum, a pair each of mandibles and maxillae, a labium, and a tonguelike hypopharynx. The type of mouthparts an insect possesses determines how it feeds. We discuss some of these modifications later.
The thorax is composed of three somites: prothorax, mesothorax, and metathorax, each bearing a pair of legs (Figure 20-4). In most insects the mesothorax and metathorax each bear a pair of wings. Wings are cuticular extensions formed by the epidermis. They consist of a double membrane containing veins of thicker cuticle that serve to strengthen the wing. Although these veins vary in their patterns among different taxa, they are constant within a family, genus, or species and serve as one means of classification and identification.
Legs of insects often are modified
for special purposes. Terrestrial forms
have walking legs with terminal pads
and claws as in beetles. These pads may be sticky for walking upside
down, as in house flies. Hindlegs of
grasshoppers and crickets are adapted
for jumping (Figure 20-6). Mole crickets
have the first pair of legs modified
for burrowing in the ground. Water
bugs and many beetles have paddleshaped
appendages for swimming. For
grasping prey, forelegs of a praying
mantis are long and strong (Figure
20-7). Legs of honey bees show
complex adaptations for collecting
pollen (Figure 20-8).
The abdomen of insects is composed of 9 to 11 segments; the eleventh, when present, is reduced to a pair of cerci (appendages at the posterior end). Larval or nymphal forms have a variety of abdominal appendages, but these appendages are lacking in adults. The end of the abdomen bears external genitalia (Figure 20-4A).
There are innumerable variations in body form among insects. Beetles are usually thick and plump (Figure 20-9A); damselflies, ant lions, and walking sticks are long and slender (Figure 20-9B); many aquatic beetles are streamlined; and cockroaches are flat, adapted to living in crevices. The ovipositor of female ichneumon wasps is extremely long (Figure 20-10). Their cerci form horny forceps in earwigs and are long and many jointed in stoneflies and mayflies. Antennae are long in cockroaches and katydids, short in dragonflies and most beetles, knobbed in butterflies, and plumed in most moths. Other variations exist (Figure 20-5).
Locomotion
Walking: When walking, most insects use a triangle of legs involving the first and last leg of one side together with the middle leg of the opposite side. In this way, insects keep three of their six legs on the ground, a tripod arrangement that bestows stability.
Some insects, such as water striders Gerris (L. gero, to carry), are able to walk on the surface of water. A water strider has on its footpads nonwetting hairs that do not break the surface film of water but merely indent it. As it skates along, Gerris uses only the two posterior pairs of legs and steers with the anterior pair (Figure 20-11). Bodies of marine water striders Halobates (Gr. halos, the sea, + baites, one that treads), excellent surfers on rough ocean waves, are further protected by a water-repellent coat of close-set hairs shaped like thick hooks.
Power of Flight: Insects share the power of flight with birds and flying mammals. However, their wings evolved in a different manner from limb buds of birds and mammals and are not homologous to them. Insect wings are formed by outgrowths from the body wall of the mesothoracic and metathoracic segments and are composed of cuticle.
Most insects have two pairs of wings, but Diptera (true flies) have only one pair, the hindwings being represented by a pair of tiny halteres (balancers) that vibrate and are responsible for equilibrium during flight. Males of order Strepsiptera have only a hind pair of wings and an anterior pair of halteres. Males of scale insects also have one pair of wings but no halteres. Some insects are wingless. Ants and termites, for example, have wings only on males, and on females during certain periods; workers are always wingless. Lice and fleas are always wingless.
Wings may be thin and membranous, as in flies and many others (Figure 20-10); thick and horny, such as the front wings of beetles (Figure 20-9A); parchmentlike, such as the front wings of grasshoppers; covered with fine scales, as in butterflies and moths; or covered with hairs, as in caddis flies.
Wing movements are controlled by a complex of muscles in the thorax. Direct flight muscles are attached to a part of the wing itself. Indirect flight muscles are not attached to the wing and cause wing movement by altering the shape of the thorax. The wing is hinged at the thoracic tergum and also slightly laterally on a pleural process, which acts as a fulcrum (Figure 20-12). In all insects, the upstroke of the wing is effected by contracting indirect muscles that pull the tergum down toward the sternum (Figure 20-12A). Dragonflies and cockroaches accomplish the downstroke by contracting direct muscles attached to the wings lateral to the pleural fulcrum. In Hymenoptera and Diptera all flight muscles are indirect. The downstroke occurs when the sternotergal muscles relax and longitudinal muscles of the thorax arch the tergum (Figure 20-12B), pulling up the tergal articulations relative to the pleura. The downstroke in beetles and grasshoppers involves both direct and indirect muscles.
Flight-muscle contraction has two basic types of neural control: synchronous and asynchronous. Larger insects such as dragonflies and butterflies have synchronous muscles, in which a single volley of nerve impulses stimulates a muscle contraction and thus one wing stroke. Asynchronous muscles are found in more specialized insects. Their mechanism of action is complex and depends on storage of potential energy in resilient parts of the thoracic cuticle. As one set of muscles contracts (moving the wing in one direction), they stretch the antagonistic set of muscles, causing them to contract (and move the wing in the other direction). Because muscle contractions are not phase-related to nervous stimulation, only occasional nerve impulses are necessary to keep the muscles responsive to alternating stretch activation. Thus extremely rapid wing beats are possible. For example, butterflies (with synchronous muscles) may beat as few as four times per second. Insects with asynchronous muscles, such as flies and bees, may vibrate at 100 beats per second or more. The fruit fly Drosophila (Gr. drosos, dew, + philos, loving) can fly at 300 beats per second, and midges have been clocked at more than 1000 beats per second.
Obviously flying entails more than a simple flapping of wings; a forward thrust is necessary. As the indirect flight muscles alternate rhythmically to raise and lower the wings, the direct flight muscles alter the angle of the wings so that they act as lifting airfoils during both the upstroke and the downstroke, twisting the leading edge of the wings downward during the downstroke and upward during the upstroke. A figure-eight movement (Figure 20-12C) results, spilling air from the trailing edges of the wings. The quality of the forward thrust depends, of course, on several factors, such as variations in wing venation, how much the wings are tilted, and how they are feathered.
Flight speeds vary. The fastest flyers usually have narrow, fast-moving wings with a strong tilt and a strong figure-eight component. Sphinx moths and horse flies achieve approximately 48 km (30 miles) per hour and dragonflies approximately 40 km (25 miles) per hour. Some insects are capable of long continuous flights. Migrating monarch butterflies, Danaus plexippus (Gr. after Danaus, mythical king of Arabia) (see Figure 20-28), travel south for hundreds of miles in the fall, flying at a speed of approximately 10 km (6 miles) per hour.
Internal Form and Function
Nutrition
The digestive system (Figure 20-13) consists of a foregut (mouth with salivary glands, esophagus, crop for storage, and gizzard for grinding in some); a midgut (stomach and gastric ceca); and a hindgut (intestine, rectum, and anus). Some digestion may take place in the crop as food mixes with enzymes from the saliva, but no absorption takes place there. The main site for digestion and absorption is the midgut, and the ceca may increase the digestive and absorptive area. Little absorption of nutrients occurs in the hindgut (with certain exceptions, such as woodeating termites), but this is a major area for resorption of water and some ions.
Most insects feed on plant juices and plant tissues (phytophagous or herbivorous). Some insects feed on specific plants; others, such as grasshoppers, will eat almost any plant. Caterpillars of many moths and butterflies eat foliage of only certain plants. Certain species of ants and termites cultivate fungus gardens as a source of food.
Many beetles and larvae of many insects live on dead animals (saprophagous). Some insects are predaceous, catching and eating other insects as well as other types of animals (Figure 20-7). However, the so-called predaceous diving beetle Cybister fimbriolatus (Gr. kybister, diver) is not as predaceous as once supposed, but is largely a scavenger.
Many insects are parasitic as adults, as larvae, or, in some cases, both juveniles and adults are parasites. For example, fleas (Figure 20-14) live on blood of mammals as adults, but their larvae are free-living scavengers. Lice (Figures 20-15 and 20-16) are parasitic throughout their life cycle. Many parasitic insects are themselves parasitized by other insects, a condition known as hyperparasitism. Larvae of many varieties of wasps live inside the bodies of spiders or other insects (Figure 20-17), consuming their hosts and eventually killing them. Because they always destroy their hosts, they are known as parasitoids (considered a particular type of parasite); typical parasites normally do not kill their hosts. Parasitoid insects are enormously important in controlling populations of other insects.
For each type of feeding, mouthparts are adapted in a specialized way. Sucking mouthparts usually form a tube and can pierce tissues of plants or animals. Water scorpions (Ranatra fusca order Hemiptera) demonstrate this arrangement well. Water scorpions are sticklike aquatic insects with a slender caudal respiratory tube. They have a beak containing four piercing, needlelike stylets made up of two mandibles and two maxillae. These parts fit together to form two tubes, a salivary tube for injecting saliva into prey and a food tube for drawing body fluid from prey. Mosquitoes also combine piercing with needlelike stylets and sucking through a food channel (Figure 20-18B). In honey bees the labium forms a flexible and contractile “tongue” covered with many hairs. When a bee plunges its proboscis into nectar, the tip of the tongue bends upward and moves back and forth rapidly. Liquid enters the tube by capillarity and is drawn inside continuously by a pumping pharynx. In butterflies and moths, mandibles are usually absent, and the maxillae form a long sucking proboscis (Figure 20-18C) for drawing nectar from flowers. At rest the proboscis coils into a flat spiral. In feeding it extends, and fluid is pumped inside by pharyngeal muscles.
House flies, blow flies, and fruit flies have sponging and lapping mouthparts (Figure 20-18D). At the apex of the labium is a pair of large, soft lobes with grooves on the lower surface that serve as food channels. These flies lap up liquid food or liquefy food first with salivary secretions. Horse flies not only sponge up surface liquids but bite into skin with slender, tapering mandibles and then sponge up blood.
Biting mouthparts such as those of grasshoppers and many other herbivorous insects are adapted for seizing and crushing food (Figure 20-18A); those of most carnivorous insects are sharp and pointed for piercing their prey. Mandibles of chewing insects are strong, toothed plates whose edges can bite or tear while the maxillae hold the food and pass it toward the mouth. Enzymes secreted by the salivary glands add chemical action to the chewing process.
Circulation
A tubular heart in the pericardial cavity (Figure 20-13) moves hemolymph (blood) forward through the only blood vessel, a dorsal aorta. The heartbeat is a peristaltic wave. Accessory pulsatory organs help move hemolymph into the wings and legs, and flow is also facilitated by various body movements. Hemolymph consists of plasma and amebocytes and apparently has little to do with oxygen transport.
Gas Exchange
Terrestrial animals require efficient respiratory systems that permit rapid oxygen and carbon dioxide exchange but at the same time restrict water loss. In insects this is the function of the tracheal system, an extensive network of thin-walled tubes that branch into every part of the body (Figure 20-19). The tracheal trunks open to the outside by paired spiracles, usually two on the thorax and seven or eight on the abdomen. A spiracle may be merely a hole in the integument, as in primary wingless insects, but there is usually a valve or some sort of closing mechanism that reduces water loss. Evolution of such a device must have been very important in enabling insects to move into drier habitats. A spiracle may also possess a filtering device such as a sieve plate or a set of interlocking bristles that may prevent entrance of water, parasites, or dust into the tracheae.
Tracheae are composed of a single layer of cells and are lined with cuticle that is shed along with the outer cuticle, during molts. Spiral thickenings of the cuticle (called taenidia) support the tracheae and prevent their collapse. Tracheae branch out into smaller tubes, ending in very fine, fluid-filled tubules called tracheoles (lined with cuticle, but not shed at ecdysis), which branch into a fine network over the cells. In large insects the largest tracheae may be several millimeters in diameter but taper down to 1 to 2 µm. Tracheoles then taper to 0.5 to 0.1 µm in diameter. In one stage of silkworm larvae it is estimated that there are 1.5 million tracheoles! Scarcely any living cell is more than a few micrometers away from a tracheole. In fact, the ends of some tracheoles actually indent the membranes of the cells they supply, so that they terminate close to mitochondria. The tracheal system affords efficient transport without use of oxygen-carrying pigments in hemolymph.
The tracheal system may also include air sacs, which are apparently dilated tracheae without taenidia (Figure 20-20A). They are thin walled and flexible and are mostly in the body cavity but also in appendages. In many insects the air sacs increase the volume of air inspired and expired. Muscular movements in the abdomen draw air into the tracheae and expand the sacs, which collapse on expiration. In some insects— locusts, for example—additional pumping is provided by telescoping the abdomen, pumping with the prothorax, or thrusting the head forward and backward. In some insects, air sacs have functions other than respiratory. For example, they may allow internal organs to change in volume during growth without changing the shape of the insect, and they reduce the weight of large insects. In some very small insects, gas transport occurs entirely by diffusion along a concentration gradient. Consumption of oxygen causes a reduced pressure in the tracheae that sucks air in through the spiracles.
The tracheal system is an adaptation for air breathing, but many insects (nymphs, larvae, and adults) live in water. In small, soft-bodied aquatic nymphs, gaseous exchange may occur by diffusion through the body wall, usually into and out of a tracheal network just under the integument. Aquatic nymphs of stoneflies and mayflies have tracheal gills, which are thin extensions of the body wall containing a rich tracheal supply. Gills of dragonfly nymphs are ridges in the rectum (rectal gills) where gas exchange occurs as water enters and leaves.
Excretion and Water Balance
Insects and spiders have a unique excretory system consisting of malpighian tubules that operate in conjunction with specialized glands in the wall of the rectum. Malpighian tubules, variable in number, are thin, elastic, blind tubules attached to the juncture between the midgut and hindgut (Figures 20-13 and 20-20A). Free ends of the tubules lie in the hemocoel and are bathed in hemolymph.
The mechanism of urine formation in malpighian tubules of herbivorous insects appears to depend on active secretion of potassium into the tubules (Figure 20-20B). This primary secretion of ions pulls water along with it by osmosis to produce a potassium-rich fluid. Other solutes and waste materials also are secreted or diffuse into the tubule. The predominant waste product of nitrogen metabolism in most insects is uric acid, which is virtually insoluble in water. Uric acid enters the upper end of the tubule, where the pH is slightly alkaline, as relatively soluble potassium and urate (abbreviated KHUr in Figure 20-20). As formative urine passes into the lower end of the tubule, potassium combines with carbon dioxide, is reabsorbed as potassium bicarbonate (KHCO3), the pH changes to acidic (pH 6.6), and insoluble uric acid (HUr) precipitates out. As urine drains into the intestine and passes through the hindgut, specialized rectal glands absorb chloride, sodium (and in some cases potassium), and water.
Since water requirements vary among different types of insects, this ability to cycle water and salts is very important. Insects living in dry environments may resorb nearly all water from the rectum, producing a nearly dry mixture of urine and feces. Leaf-feeding insects ingest and excrete quantities of fluid. Freshwater larvae need to excrete water and conserve salts. Insects that feed on dry grains need to conserve water and excrete salt.
Nervous System
The nervous system in general resembles that of larger crustaceans, with a similar tendency toward fusion of ganglia (Figure 20-13). A number of insects have a giant fiber system. There is also a stomodeal nervous system that corresponds in function to the autonomic nervous system of vertebrates. Neurosecretory cells located in various parts of the brain have an endocrine function, but, except for their role in molting and metamorphosis, little is known of their activity.
Sense Organs
Along with neuromuscular coordination, insects have unusually keen sensory perception. Their sense organs are mostly microscopic and are located chiefly in the body wall. Each type usually responds to a specific stimulus. The various organs are receptive to mechanical, auditory, chemical, visual, and other stimuli.
Mechanoreception: Mechanical stimuli, those involving touch, pressure, and vibration, are picked up by sensilla. A sensillum may be simply a seta, or hairlike process, connected with a nerve cell, a nerve ending just under the cuticle and lacking a seta, or a more complex organ (scolopophorous organ) consisting of sensory cells with their endings attached to the body wall. Such organs are widely distributed over the antennae, legs, and body.
Auditory Reception: Very sensitive setae (hair sensilla) or tympanal organs may detect airborne sounds. In tympanal organs a number of sensory cells (ranging from a few to hundreds) extend to a very thin tympanic membrane that encloses an air space in which vibrations can be detected. Tympanal organs occur in certain Orthoptera (Figure 20-4), Homoptera, and Lepidoptera. Some insects are fairly insensitive to airborne sounds but can detect vibrations reaching them through the substrate. Organs on the legs usually detect vibrations of the substrate.
Chemoreception: Chemoreceptors (for taste or smell) are usually bundles of sensory cell processes often located in sensory pits. These are often on mouthparts, but in ants, bees, and wasps they are also on the antennae, and butterflies, moths, and flies also have them on the legs. Chemical sense is generally keen, and some insects can detect certain odors for several kilometers. Many patterns of insect behavior such as feeding, mating, habitat selection, and host-parasite relations are mediated through chemical senses. These senses play a crucial role in responses of insects to artificial repellents and attractants.
Visual Reception: Insect eyes are of two types, simple and compound. Simple eyes are found in some nymphs and larvae and in many adults. Most insects have three ocelli on the head. Honey bees probably use ocelli to monitor light intensity but not to form images.
Most adult insects have compound eyes, which may cover much of the head. They consist of thousands of ommatidia—6300 in the eye of a honey bee, for example. The structure of the compound eye is similar to that of crustaceans (Figure 20-21). An insect such as a honey bee can see simultaneously in almost all directions around its body, but it is more myopic than humans, and images, even of nearby objects, are fuzzy. However, most flying insects rate much higher than humans in flicker-fusion tests. Flickers of light become fused in human eyes at a frequency of 45 to 55 per second, but bees and blow flies can distinguish as many as 200 to 300 separate flashes of light per second. This is probably an advantage in analyzing a fastchanging landscape during flight.
A bee can distinguish colors, but its sensitivity begins in the ultraviolet range, which human eyes cannot see, and extends into the orange; honey bees cannot distinguish shades of red from shades of gray.
Other Senses: Insects also have welldeveloped senses for temperature, especially on the antennae and legs, and for humidity, proprioception (sensation of muscle stretch and body position), gravity, and other physical properties.
Neuromuscular Coordination
Insects are active creatures with excellent neuromuscular coordination. Arthropod muscles are typically cross-striated, just as vertebrate skeletal muscles are. A flea can leap a distance of 100 times its own length, and an ant can carry in its jaws a load greater than its own weight. This sounds as though insect muscle were stronger than that of other animals. Actually, however, the force a particular muscle can exert is related directly to its cross-sectional area, not its length. Based on maximum load moved per square centimeter of cross section, the strength of insect muscle is relatively the same as that of vertebrate muscle. The illusion of great strength of insects (and other small animals) is simply a consequence of small body size.
Reproduction
Sexes are separate in insects, and fertilization is usually internal. Parthenogenesis occurs prominently in Homoptera and Hymenoptera. Insects have various means of attracting mates. A female moth releases a powerful pheromone that males can detect for a great distance. Fireflies use flashes of light; some insects find each other by sounds or color signals and by various kinds of courtship behavior.
Males usually deposit sperm in a female’s vagina during copulation (Figures 20-13 and 20-22). In some orders sperm are encased in spermatophores that may be transferred at copulation or deposited on the substratum to be picked up by a female. A male silverfish deposits a spermatophore on the ground, then spins signal threads to guide a female to it. During evolutionary transition of ancestral insects from aquatic to terrestrial life, spermatophores were used widely and copulation evolved much later.
Usually sperm are stored in the spermatheca of a female in numbers sufficient to fertilize more than one batch of eggs. Many insects mate only once during their lifetime, but male damselflies copulate several times per day.
Insects usually lay a great many eggs. A queen honey bee, for example, may lay more than 1 million eggs during her lifetime. On the other hand, some flies are viviparous and bring forth only a single offspring at a time. Insects that make no provision for care of their young may lay many more eggs than do insects that provide for their young or those that have a very short life cycle.
Most species lay their eggs in a particular habitat to which visual, chemical, or other cues guide them. Butterflies and moths lay their eggs on the specific kind of plant on which the caterpillar must feed. A tiger moth may look for a pigweed, a sphinx moth for a tomato or tobacco plant, and a monarch butterfly for a milkweed plant (Figure 20-23). Insects whose immature stages are aquatic characteristically lay their eggs in water (Figure 20-24). A tiny braconid wasp lays her eggs on the caterpillar of the sphinx moth where they will feed and pupate in tiny white cocoons (Figure 20-17). An ichneumon wasp, with unerring accuracy, seeks out a certain kind of larva in which her young will live as parasitoids. Her long ovipositors may have to penetrate 1 to 2 cm of wood to find a larva of a wood wasp or a wood-boring beetle in which she will deposit her eggs (Figure 20-10).
Metamorphosis and Growth
Early development occurs within the egg, and hatching young escape from the egg in various ways. During postembryonic development most insects change in form, undergoing metamorphosis (Figure 20-23). During this period they must undergo a series of molts to grow, and each stage between molts is called an instar.
Although metamorphosis occurs in many animals, insects illustrate it more dramatically than any other group. The transformation, for instance, of a hickory horned devil caterpillar into a beautiful royal walnut moth represents an astonishing morphological change. In insects metamorphosis is associated with evolution of wings, which are restricted to the reproductive stage where they are most beneficial.
Holometabolous
Metamorphosis
Approximately 88% of insects undergo a holometabolous (Gr. holo, complete, + metabole, change) metamorphosis, which separates physiological processes of growth (larva) from those of differentiation (pupa) and reproduction (adult) (Figure 20-23). Each stage functions efficiently without competition with the other stages, for larvae often live in entirely different surroundings and eat different foods from adults. The wormlike larvae, which usually have chewing mouthparts, are known as caterpillars, maggots, bagworms, fuzzy worms, or grubs. After a series of instars a larva forms a case or cocoon around itself and becomes a pupa, or chrysalis, a nonfeeding stage in which many insects pass the winter. When the final molt occurs over winter, the full-grown adult emerges, pale and with wings wrinkled. In a short time the wings expand and harden, and the insect is on its way. The stages, then, are egg, larva (several instars), pupa, and adult (Figure 20-23). Adults undergo no further molting.
Hemimetabolous
Metamorphosis
Some insects undergo a hemimetabolous (Gr. hemi, half, + metabole, change), or gradual (incomplete), metamorphosis. These include grasshoppers, cicadas, mantids, and terrestrial bugs, which have terrestrial young, and mayflies, stoneflies, dragonflies, and aquatic bugs that lay their eggs in water and whose young are aquatic. The young are called nymphs, and their wings develop externally as budlike outgrowths in early instars and increase in size as the animal grows by successive molts and becomes a winged adult (Figures 20-25 and 20-26). Aquatic nymphs in some orders have tracheal gills or other modifications for aquatic life (Figure 20-27). The stages are egg, nymph (several instars), and adult (Figure 20-26).
Direct Development
A few insects, such as silverfish and springtails, undergo direct development. The young, or juveniles, are similar to adults except in size and sexual maturation. The stages are egg, juvenile, and adult. Such insects include the primitively wingless insects.
Physiology of Metamorphosis
Hormones regulate metamorphosis in insects. Major endocrine organs that are involved in development are the brain, the prothoracic (ecdysial) glands, the corpora cardiaca, and the corpora allata (Figure 36-4).
The intercerebral part of the brain and the ganglia of the nerve cord contain several groups of neurosecretory cells that produce a brain hormone called prothoracicotropic hormone (PTTH). These neurosecretory cells send their axons to paired organs behind the brain, the corpora cardiaca, which serve as storage and release organs for PTTH (and also produce other hormones). PTTH is carried in the hemolymph to the prothoracic gland, a glandular organ that in the head or prothorax produces molting hormone, or ecdysone (ek´duhsone) in response to brain hormone. Ecdysone sets in motion certain processes that lead to casting off of the old cuticle (ecdysis).
Simple molting persists as long as juvenile hormone is present in sufficient amounts, along with molting hormone in the hemolymph, and each molt produces a larger larva. The corpora allata produce juvenile hormone (Figure 36-4).
In later instars, the corpora allata release progressively less juvenile hormone. When juvenile hormone is at a very low level, a larva molts to become a pupa, and cessation of juvenile hormone production in the pupa leads to an adult at the next molt (metamorphosis). Control of development is the same in hemimetabolous insects, except that there is no pupa, and cessation of juvenile hormone production occurs in the final nymphal instar. The corpora allata again become active in adult insects, in which juvenile hormone is important in normal egg production. The prothoracic glands degenerate in adults of most insects, and adults do not molt. Insect hormones have received much interesting experimental study.
For example, if the corpora allata (and thus juvenile hormone) are removed surgically from a larva, the following molt will result in metamorphosis. Conversely, if the corpora allata from a young larva are transplanted into a final larval instar, the latter can be converted into a giant larva, because metamorphosis to the pupa cannot occur.
Diapause
Many animals, including many types of insects, undergo a period of dormancy in their annual life cycle. In temperate zones there may be a period of winter dormancy, called hibernation, or a period of summer dormancy, called estivation, or both. There are periods in the life cycle of many insects when eggs, larvae, pupae, or even adults remain dormant for a long time because external conditions are too harsh or unfavorable for survival in states of normal activity. Thus the life cycle is synchronized with periods of suitable environmental conditions and abundance of food. Most insects enter a dormant state when some factor of the environment, such as temperature, becomes unfavorable, and dormancy continues until conditions again become favorable.
However, some species have a prolonged arrest of growth that occurs regardless of environment, whether or not favorable conditions prevail. This type of dormancy is called diapause (di´a-poz) (Gr. dia, through, dividing into two parts, + pausis, a stopping), and it is an important adaptation to survive adverse environmental conditions. Diapause is genetically determined in each species and sometimes varies between strains within a species, but it is usually initiated by a particular signal. In the environment of an insect, such signals forecast adverse conditions to come, for example, lengthening or shortening of the days. Thus photoperiod, or day length, is often the signal that initiates diapause. After diapause is initiated, another environmental signal is usually required to end it. Such signal may be return of favorable temperature after a prolonged period of cold or an occasion of rain after a dry period, as in a desert.
Diapause always occurs at the end of an active growth stage of the molting cycle so that, when the diapause period is over, the insect is ready for another molt. One species of the ant Myrmica reaches the third instar stage in late summer. Many larvae do not develop beyond this point until the following spring, even if temperatures are mild or if the larvae are kept in a warm laboratory.
Defense
Insects as a group display many colors. This is especially true of butterflies, moths, and beetles. Even within a species the color pattern may vary in a seasonal way, and there also may be color differences between males and females. Some of the color patterns in insects are probably highly adaptive, such as those for protective coloration, warning coloration, and mimicry (Figures 20-28 and 20-29).
Besides color, insects have other methods of protecting themselves. The cuticular exoskeleton affords good protection for many of them. Some, such as stink bugs, have repulsive odors and tastes; other protect themselves by a good offense, for many are very aggressive and fight (for example, bees and ants); and still others are swift in running for cover when danger threatens.
Many insects practice chemical warfare in a variety of ingenious ways. Some repel an assault by virtue of their bad taste, odor, or poisonous properties; others use chemical exudates that mechanically prevent a predator from attacking. Caterpillars of some monarch butterflies (Figure 20-28) assimilate cardiac glycosides from certain species of milkweed (family Asclepiadaceae); this substance confers unpalatability on the butterflies after metamorphosis and induces vomiting in their predators. Bombardier beetles, on the other hand, produce an irritating spray, which they aim accurately at attacking ants or other enemies.
Behavior and Communication
The keen sensory perceptions of insects make them extremely responsive to many stimuli. Stimuli may be internal (physiological) or external (environmental), and responses are governed by both the physiological state of the animal and the pattern of nerve pathways traveled by the impulses. Many responses are simple, such as orientation toward or away from the stimulus, for example, avoidance of light by a cockroach, or attraction of carrion flies to the odor of dead flesh.
Much behavior of insects, however, is not a simple matter of orientation but involves a complex series of responses. A pair of tumble bugs, or dung beetles, chew off a bit of dung, roll it into a ball, and roll the ball laboriously to where they intend to bury it, after laying their eggs in it (Figure 20-30). Cicadas slit the bark of a twig and then lay an egg in each of the slits. Female potter wasps Eumenes scoop up clay into pellets, carry them one by one to a building site, and fashion them into dainty little narrownecked clay pots, into each of which the wasps lay an egg. Then a mother wasp hunts and paralyzes a number of caterpillars, pokes them into the opening of a pot, and closes up the opening with clay. Each egg, in its own protective pot, hatches to find a well-stocked larder of food.
Much of such behavior is innate; however, a great deal more learning is involved than was once believed. The potter wasp, for example, must learn where she has left her pots if she is to return to fill them with caterpillars one at a time. Social insects, which have been studied extensively, are capable of most of the basic forms of learning used by mammals. An exception is insight learning. Apparently insects, when faced with a new problem, cannot reorganize their memories to construct a new response.
Insects communicate with each other by means of chemical, visual, auditory, and tactile signals. Chemical signals take the form of pheromones, which are substances secreted by one individual that affect the behavior or physiological processes of another individual. Many pheromones have been described. Like hormones, pheromones are effective in minute quantities. Known functions of various pheromones include attraction of the opposite sex, release of certain behavior patterns (for example, aggregation pheromones to enable mass attack of bark beetles on a tree or for overwintering of ladybird beetles), to fend off aggression, to mark trails and territories, and to signal alarms. Social insects, such as bees, ants, wasps, and termites, can recognize a nestmate—or an alien in the nest—by means of identification pheromones. Social parasites escape detection—and certain destruction—by imitating or duplicating pheromones produced by members of their host colony. Pheromones determine caste in termites and to some extent in ants and bees. They are a primary integrating force in populations of social insects.
Many insect pheromones have been isolated and identified. Traps baited with pheromones have been used for many years to monitor insects of economic importance. They can be used to detect the presence of an insect, such as a new arrival from a neighboring area (tracking spread of European gypsy moth in the United States or the presence of European corn ear worms in a field), or to monitor changes in population levels. Use of pheromone traps has become an important tool to detect potential outbreaks, allowing sufficient time to plan remedial action.
Sound production and reception (phonoproduction and phonoreception) in insects have been studied extensively, and although a sense of hearing is not present in all insects, this means of communication is meaningful to those insects that use it. Sounds serve as warning devices, advertisement of territorial claims, or courtship songs. Sounds of crickets and grasshoppers seem to be concerned with courtship and aggression. Male crickets scrape the modified edges of their forewings together to produce their characteristic chirping. The long, drawn-out sound of male cicadas, a call to attract females, is produced by vibrating membranes in a pair of organs on the ventral side of the basal abdominal segment.
There are many forms of tactile communication, such as tapping, stroking, grasping, and antennae touching, which evoke responses varying from recognition to recruitment and alarm. Certain kinds of flies, springtails, and beetles manufacture their own visual signals in the form of bioluminescence. Best known of a luminescent beetles are fireflies, or lightningbugs (which are neither flies nor bugs, but beetles), in which the flash of light helps to locate a prospective mate. Each species has its own characteristic flashing rhythm produced on the ventral side of the last abdominal segments. Females flash an answer to the species-specific pattern to attract males. This interesting “love call” has been adopted by species of Photuris, which prey on the male fireflies of other species they attract (Figure 20-31).
Social Behavior
Insects rank very high in the animal kingdom in their organization of social groups, and cooperation within the more complex groups depends heavily on chemical and tactile communication. Social communities are not all complex, however. Some community groups are temporary and uncoordinated, as are hibernating associations of carpenter bees or feeding gatherings of aphids. Some are coordinated for only brief periods, and some cooperate more fully, such as tent caterpillars Malacosoma, which join in building a home web and feeding net. However, all of these are open communities with limited social behavior.
In the true societies of some orders, such as Hymenoptera (honey bees and ants) and Isoptera (termites), a complex social life is necessary for perpetuation of the species. They involve all stages of the life cycle, communities are usually permanent, all activities are collective, and there is reciprocal communication and division of labor. The society usually demonstrates polymorphism, or caste differentiation.
Honey bees have one of the most complex organizations in the insect world. Instead of lasting one season, their organization continues for an indefinite period. As many as 60,000 to 70,000 honey bees may live in a single hive. Of these, there are three castes: a single sexually mature female, or queen; a few hundred drones, which are sexually mature males; and the rest are workers, which are sexually inactive genetic females (Figure 20-32).
Workers take care of the young, secrete wax with which they build the six-sided cells of the honeycomb, gather nectar from flowers, manufacture honey, collect pollen, and ventilate and guard the hive. One drone, sometimes more, fertilizes the queen during the mating flight, at which time enough sperm is stored in her spermatheca to last her a lifetime.
Castes are determined partly by fertilization and partly by what is fed to larvae. Drones develop parthenogenetically from unfertilized eggs (and consequently are haploid); queens and workers develop from fertilized eggs (and thus are diploid; see haplodiploidy). Female larvae that will become queens are fed royal jelly, a secretion from the salivary glands of nurse workers. Royal jelly differs from the “worker jelly” fed to ordinary larvae, but components in it that are essential for queen determination have not yet been identified. Honey and pollen are added to worker diet about the third day of larval life. Pheromones in “queen substance,” which is produced by the queen’s mandibular glands, prevent female workers from maturing sexually. Workers produce royal jelly only when the level of “queen substance” pheromone in the colony drops. This change occurs when the queen becomes too old, dies, or is removed. Then workers’ ovaries develop, and they start enlarging a larval cell and feeding a larva royal jelly to produce a new queen. Honey bees have evolved an efficient system of communication by which, through certain body movements, their scouts inform workers of the location and quantity of food sources (Figure 38-23)
Termite colonies contain several castes, consisting of fertile individuals, both males and females, and sterile individuals (Figure 20-33). Some fertile individuals may have wings and may leave the colony, mate, lose their wings, and as king and queen start a new colony. Wingless fertile individuals may under certain conditions substitute for the king or queen. Sterile members are wingless and become workers and soldiers. Soldiers have large heads and mandibles and serve for defense of the colony. As in bees and ants, extrinsic factors cause caste differentiation. Reproductive individuals and soldiers secrete inhibiting pheromones that pass throughout the colony to nymphs through a mutual feeding process, called trophallaxis, so that they become sterile workers. Workers also produce pheromones, and if the level of “worker substance” or “soldier substance” falls, as might happen after an attack by marauding predators, for example, the next generation produces compensating proportions of the appropriate caste.
Ants also have highly organized societies. Superficially, they resemble termites, but they are quite different (belong to a different order) and can be distinguished easily. In contrast to termites, ants are usually dark in color, are hard bodied, and have a constriction posterior to their first abdominal somite.
In ant colonies males die soon after mating and the queen either starts her own new colony or joins some established colony and does the egg laying. Sterile females are wingless workers and soldiers that do the work of the colony: gather food, care for the young, and protect the colony. In many larger colonies there may be two or three types of individuals within each caste.
Ants have evolved some striking patterns of “economic” behavior, such as making slaves, farming fungi, herding “ant cows” (aphids or other homopterans) (Figure 20-34A), sewing their nests together with silk (Figure 20-34B), and using tools.
Insecta (L. insectus, cut into) are the most diverse and abundant of all groups of arthropods. There are more species of insects than species of all other classes of animals combined. The number of insect species has been estimated at up to 10 million. There is also striking evidence of continuing evolution among insects at the present time, even though the fossil record indicates that the group as a whole is stable.
It is difficult to appreciate fully the significance of this extensive group and its role in the biological pattern of animal life. The study of insects (entomology) occupies the time and resources of skilled men and women all over the world. The struggle between humans and their insect competitors seems to be endless, yet paradoxically insects have so interwoven themselves into the economy of nature in so many useful roles that we would have a difficult time without them.
Insects differ from other arthropods in having three pairs of legs and usually two pairs of wings on the thoracic region of the body, although some have one pair of wings or none. Insects range in size from less than 1 mm to 20 cm in length, the majority being less than 2.5 cm long. Generally, the largest insects live in tropical areas.
Distribution
Insects are among the most abundant and widespread of all land animals. They have spread into practically all habitats that will support life except most of the sea. Relatively few are marine. Marine water striders (Halobates), which live on the surface of the ocean, are the only marine invertebrates that live on the sea-air interface. Insects are common in brackish water, in salt marshes, and on sandy beaches. They are abundant in fresh water, in soil, in forests (especially the tropical forest canopy), and in plants, and they are found even in deserts and wastelands, on mountaintops, and as parasites in and on plants and animals.
Their wide distribution is made possible by their powers of flight and their highly adaptable nature. In most cases they can easily surmount barriers that are virtually impassable to many other animals. Their small size allows them to be carried by currents of both wind and water to far regions. Their well-protected eggs can withstand rigorous conditions and can be carried long distances by birds and other animals. Their agility and aggressiveness enable them to fight for every possible niche in a habitat. No single pattern of biological adaptation can be applied to them.
Adaptability
Insects, during their evolution, have shown an amazing adaptability, as evidenced by their wide distribution and enormous diversity of species. Most of their structural modifications have taken place in wings, legs, antennae, mouthparts, and alimentary canal. Such wide diversity enables this vigorous group to take advantage of all available food and shelter. Some are parasitic, some suck the sap of plants, some chew the foliage of plants, some are predaceous, and some live on the blood of various animals. Within these different groups, specialization occurs, so that a particular kind of insect will eat, for instance, leaves of only one kind of plant. This specificity of eating habits lessens competition with other species and to a great extent accounts for their biological diversity.
Insects are well adapted to dry and desert regions. The hard and protective exoskeleton helps prevent evaporation. Some insects also extract most of the water from food, fecal material, and by-products of cell metabolism.
As in other arthropods, the exoskeleton is made up of a complex system of plates known as sclerites, connected by concealed, flexible hinge joints. Muscles between sclerites enable insects to make precise movements. Rigidity of their exoskeleton is attributable to the unique scleroproteins and not to its chitin component. Its lightness makes flying possible. By contrast, the cuticle of crustaceans is stiffened mostly by minerals.
External Form and Function
Insects show a remarkable variety of morphological characteristics, but they are much more homogeneous in tagmatization than are Crustacea. Some insects are fairly generalized in body structure; some are highly specialized. Grasshoppers, or locusts, are a generalized type often used in laboratories to demonstrate the general features of insects (Figure 20-4).
Insect tagmata are head, thorax, and abdomen. The cuticle of each body somite typically is composed of four plates (sclerites), a dorsal notum (tergum), a ventral sternum, and a pair of lateral pleura. Pleura of abdominal segments are membranous rather than sclerotized.
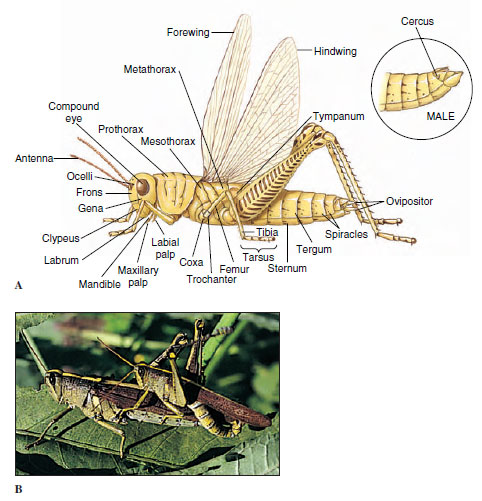 |
| Figure 20-4 A, External features of a female grasshopper. The terminal segment of a male with external genitalia is shown in inset. B, A pair of grasshoppers, Schistocerca obscura (order Orthoptera), copulating. The African desert locust mentioned in the section prologue is Schistocerca gregaria. |
The head usually bears a pair of relatively large compound eyes, a pair of antennae, and usually three ocelli. Antennae, which vary greatly in size and form (Figure 20-5), act as tactile organs, olfactory organs, and in some cases as auditory organs. Mouthparts, formed from specially hardened cuticle, typically consist of a labrum, a pair each of mandibles and maxillae, a labium, and a tonguelike hypopharynx. The type of mouthparts an insect possesses determines how it feeds. We discuss some of these modifications later.
The thorax is composed of three somites: prothorax, mesothorax, and metathorax, each bearing a pair of legs (Figure 20-4). In most insects the mesothorax and metathorax each bear a pair of wings. Wings are cuticular extensions formed by the epidermis. They consist of a double membrane containing veins of thicker cuticle that serve to strengthen the wing. Although these veins vary in their patterns among different taxa, they are constant within a family, genus, or species and serve as one means of classification and identification.
 |
 |
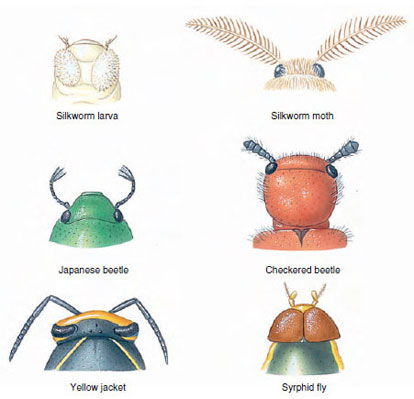
| Figure 20-5 A few of the various types of insect antennae. |
Figure 20-7 A, Praying mantis (order Orthoptera) feeding on an insect. B, Praying mantis laying eggs. |
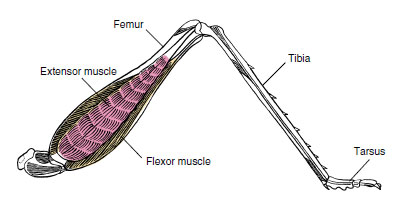 |
| Figure 20-6 Hindleg of grasshopper. Muscles that operate the leg are found within a hollow cylinder of exoskeleton. Here they are attached to the internal wall, from which they manipulate segments of limb on the principle of a lever. Note pivot joint and attachment of tendons of extensor and flexor muscles, which act reciprocally to extend and flex the limb. |
The abdomen of insects is composed of 9 to 11 segments; the eleventh, when present, is reduced to a pair of cerci (appendages at the posterior end). Larval or nymphal forms have a variety of abdominal appendages, but these appendages are lacking in adults. The end of the abdomen bears external genitalia (Figure 20-4A).
There are innumerable variations in body form among insects. Beetles are usually thick and plump (Figure 20-9A); damselflies, ant lions, and walking sticks are long and slender (Figure 20-9B); many aquatic beetles are streamlined; and cockroaches are flat, adapted to living in crevices. The ovipositor of female ichneumon wasps is extremely long (Figure 20-10). Their cerci form horny forceps in earwigs and are long and many jointed in stoneflies and mayflies. Antennae are long in cockroaches and katydids, short in dragonflies and most beetles, knobbed in butterflies, and plumed in most moths. Other variations exist (Figure 20-5).
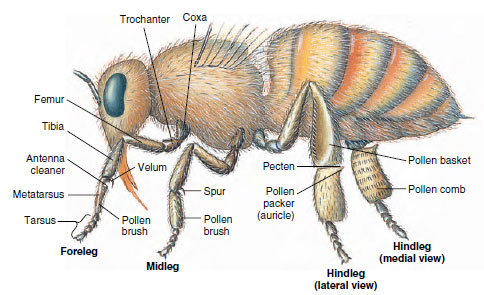 |
| Figure 20-8 Adaptive legs of worker honey bee. In the foreleg, the toothed indentation covered with the velum combs out the antennae. The spur on the middle leg removes wax from wax glands on the abdomen. Pollen brushes on the front and middle legs comb off pollen picked up on body hairs and deposit it on the pollen brushes of the hindlegs. Long hairs of the pecten on the hindleg remove pollen from the brush of the opposite leg; then the auricle (pollen packer) presses it into a pollen basket when the leg joint is flexed back. A bee carries her load in both baskets to the hive and pushes pollen into a cell, to be cared for by other workers. |
 |
 |
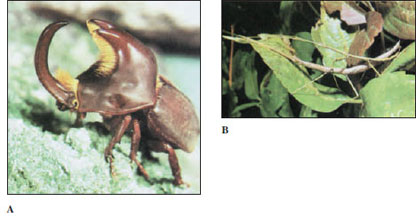
| Figure 20-9 A, A giant horned beetle Diloboderus abderus (order Coleoptera) from Uruguay. Though the ferociouslooking processes from the head and thorax might appear to be for pinching or stabbing an opponent, they actually are used to lift or pry up a rival of the same species away from resources. B, Walking sticks Diapheromera femorata (order Orthoptera), mating. The species is common in much of North America. It is wingless, and despite its camouflage as a twig, it is eaten by numerous predators. |
Figure 20-10 An ichneumon wasp with the end of the abdomen raised to thrust her long ovipositor into wood to find a tunnel made by the larva of a wood wasp or wood-boring beetle. She can bore 13 mm or more into the wood to lay her eggs in the larva of a wood-boring beetle, which will become host for the ichneumon larvae. Other ichneumon species attack spiders, moths, flies, crickets, caterpillars, and other insects. |
Locomotion
Walking: When walking, most insects use a triangle of legs involving the first and last leg of one side together with the middle leg of the opposite side. In this way, insects keep three of their six legs on the ground, a tripod arrangement that bestows stability.
 |
| Figure 20-11 Water strider, Gerris sp. (order Hemiptera). The animal is supported on its long, slender legs by the water’s surface tension. |
Some insects, such as water striders Gerris (L. gero, to carry), are able to walk on the surface of water. A water strider has on its footpads nonwetting hairs that do not break the surface film of water but merely indent it. As it skates along, Gerris uses only the two posterior pairs of legs and steers with the anterior pair (Figure 20-11). Bodies of marine water striders Halobates (Gr. halos, the sea, + baites, one that treads), excellent surfers on rough ocean waves, are further protected by a water-repellent coat of close-set hairs shaped like thick hooks.
Power of Flight: Insects share the power of flight with birds and flying mammals. However, their wings evolved in a different manner from limb buds of birds and mammals and are not homologous to them. Insect wings are formed by outgrowths from the body wall of the mesothoracic and metathoracic segments and are composed of cuticle.
Most insects have two pairs of wings, but Diptera (true flies) have only one pair, the hindwings being represented by a pair of tiny halteres (balancers) that vibrate and are responsible for equilibrium during flight. Males of order Strepsiptera have only a hind pair of wings and an anterior pair of halteres. Males of scale insects also have one pair of wings but no halteres. Some insects are wingless. Ants and termites, for example, have wings only on males, and on females during certain periods; workers are always wingless. Lice and fleas are always wingless.
Wings may be thin and membranous, as in flies and many others (Figure 20-10); thick and horny, such as the front wings of beetles (Figure 20-9A); parchmentlike, such as the front wings of grasshoppers; covered with fine scales, as in butterflies and moths; or covered with hairs, as in caddis flies.
Wing movements are controlled by a complex of muscles in the thorax. Direct flight muscles are attached to a part of the wing itself. Indirect flight muscles are not attached to the wing and cause wing movement by altering the shape of the thorax. The wing is hinged at the thoracic tergum and also slightly laterally on a pleural process, which acts as a fulcrum (Figure 20-12). In all insects, the upstroke of the wing is effected by contracting indirect muscles that pull the tergum down toward the sternum (Figure 20-12A). Dragonflies and cockroaches accomplish the downstroke by contracting direct muscles attached to the wings lateral to the pleural fulcrum. In Hymenoptera and Diptera all flight muscles are indirect. The downstroke occurs when the sternotergal muscles relax and longitudinal muscles of the thorax arch the tergum (Figure 20-12B), pulling up the tergal articulations relative to the pleura. The downstroke in beetles and grasshoppers involves both direct and indirect muscles.
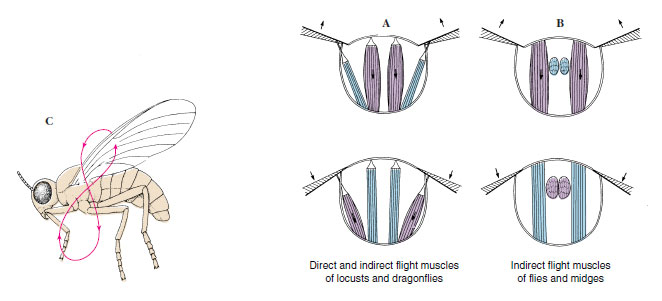 |
| Figure 20-12 A, Flight muscles of insects such as cockroaches, in which upstroke is by indirect muscles and downstroke is by direct muscles. B, In insects such as flies and bees, both upstroke and downstroke are by indirect muscles. C, The figure-eight path followed by the wing of a flying insect during the upstroke and downstroke. |
Flight-muscle contraction has two basic types of neural control: synchronous and asynchronous. Larger insects such as dragonflies and butterflies have synchronous muscles, in which a single volley of nerve impulses stimulates a muscle contraction and thus one wing stroke. Asynchronous muscles are found in more specialized insects. Their mechanism of action is complex and depends on storage of potential energy in resilient parts of the thoracic cuticle. As one set of muscles contracts (moving the wing in one direction), they stretch the antagonistic set of muscles, causing them to contract (and move the wing in the other direction). Because muscle contractions are not phase-related to nervous stimulation, only occasional nerve impulses are necessary to keep the muscles responsive to alternating stretch activation. Thus extremely rapid wing beats are possible. For example, butterflies (with synchronous muscles) may beat as few as four times per second. Insects with asynchronous muscles, such as flies and bees, may vibrate at 100 beats per second or more. The fruit fly Drosophila (Gr. drosos, dew, + philos, loving) can fly at 300 beats per second, and midges have been clocked at more than 1000 beats per second.
Obviously flying entails more than a simple flapping of wings; a forward thrust is necessary. As the indirect flight muscles alternate rhythmically to raise and lower the wings, the direct flight muscles alter the angle of the wings so that they act as lifting airfoils during both the upstroke and the downstroke, twisting the leading edge of the wings downward during the downstroke and upward during the upstroke. A figure-eight movement (Figure 20-12C) results, spilling air from the trailing edges of the wings. The quality of the forward thrust depends, of course, on several factors, such as variations in wing venation, how much the wings are tilted, and how they are feathered.
Flight speeds vary. The fastest flyers usually have narrow, fast-moving wings with a strong tilt and a strong figure-eight component. Sphinx moths and horse flies achieve approximately 48 km (30 miles) per hour and dragonflies approximately 40 km (25 miles) per hour. Some insects are capable of long continuous flights. Migrating monarch butterflies, Danaus plexippus (Gr. after Danaus, mythical king of Arabia) (see Figure 20-28), travel south for hundreds of miles in the fall, flying at a speed of approximately 10 km (6 miles) per hour.
Internal Form and Function
Nutrition
The digestive system (Figure 20-13) consists of a foregut (mouth with salivary glands, esophagus, crop for storage, and gizzard for grinding in some); a midgut (stomach and gastric ceca); and a hindgut (intestine, rectum, and anus). Some digestion may take place in the crop as food mixes with enzymes from the saliva, but no absorption takes place there. The main site for digestion and absorption is the midgut, and the ceca may increase the digestive and absorptive area. Little absorption of nutrients occurs in the hindgut (with certain exceptions, such as woodeating termites), but this is a major area for resorption of water and some ions.
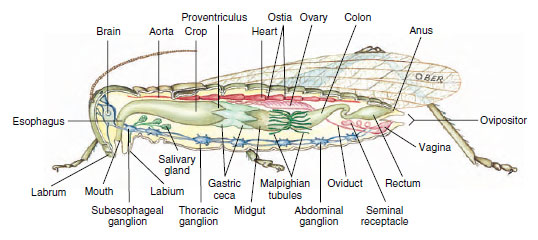 |
| Figure 20-13 Internal structure of female grasshopper. |
Most insects feed on plant juices and plant tissues (phytophagous or herbivorous). Some insects feed on specific plants; others, such as grasshoppers, will eat almost any plant. Caterpillars of many moths and butterflies eat foliage of only certain plants. Certain species of ants and termites cultivate fungus gardens as a source of food.
Many beetles and larvae of many insects live on dead animals (saprophagous). Some insects are predaceous, catching and eating other insects as well as other types of animals (Figure 20-7). However, the so-called predaceous diving beetle Cybister fimbriolatus (Gr. kybister, diver) is not as predaceous as once supposed, but is largely a scavenger.
Many insects are parasitic as adults, as larvae, or, in some cases, both juveniles and adults are parasites. For example, fleas (Figure 20-14) live on blood of mammals as adults, but their larvae are free-living scavengers. Lice (Figures 20-15 and 20-16) are parasitic throughout their life cycle. Many parasitic insects are themselves parasitized by other insects, a condition known as hyperparasitism. Larvae of many varieties of wasps live inside the bodies of spiders or other insects (Figure 20-17), consuming their hosts and eventually killing them. Because they always destroy their hosts, they are known as parasitoids (considered a particular type of parasite); typical parasites normally do not kill their hosts. Parasitoid insects are enormously important in controlling populations of other insects.
 |
 |
 |
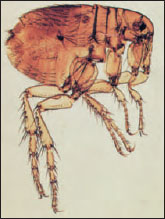
| Figure 20-14 Female human flea, Pulex irritans (order Siphonaptera). |
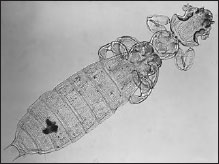
Figure 20-15 Gliricola porcelli (order Mallophaga), a chewing louse of guinea pigs. Antennae are normally held in the deep grooves on the sides of the head. |
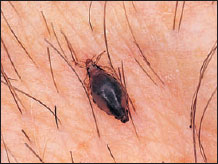
Figure 20-16 The head and body louse of humans Pediculus humanus (order Anoplura) feeding. |
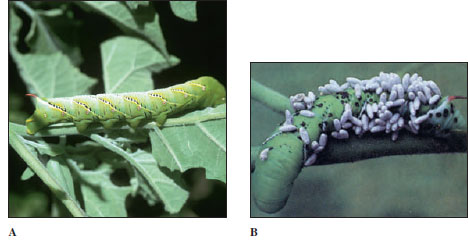 |
| Figure 20-17 A, Hornworm, larval stage of a sphinx moth (order Lepidoptera). The more than 100 species of North American sphinx moths are strong fliers and mostly nocturnal feeders. Their larvae, called hornworms because of the large, fleshy posterior spine, are often pests of tomatoes, tobacco, and other plants. B, Hornworm parasitized by a tiny wasp Apanteles (a parasitoid), which laid its eggs inside the caterpillar. The wasp larvae have emerged, and their pupae are on the caterpillar’s skin. Young wasps emerge in 5 to 10 days, but the caterpillar usually dies. |
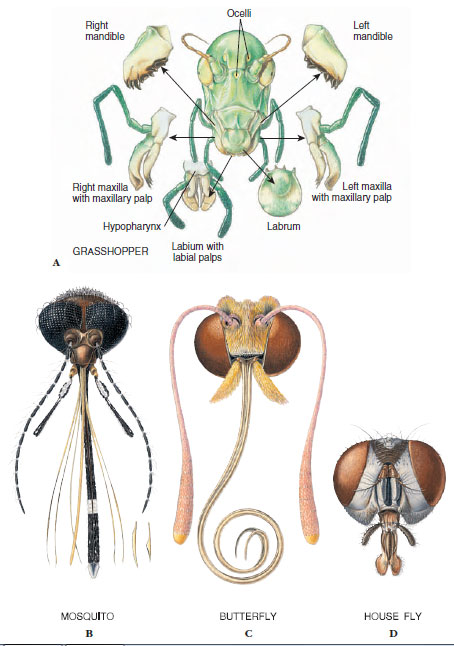 |
| Figure 20-18 Four types of insect mouthparts. (See text for description of types and examples.) |
For each type of feeding, mouthparts are adapted in a specialized way. Sucking mouthparts usually form a tube and can pierce tissues of plants or animals. Water scorpions (Ranatra fusca order Hemiptera) demonstrate this arrangement well. Water scorpions are sticklike aquatic insects with a slender caudal respiratory tube. They have a beak containing four piercing, needlelike stylets made up of two mandibles and two maxillae. These parts fit together to form two tubes, a salivary tube for injecting saliva into prey and a food tube for drawing body fluid from prey. Mosquitoes also combine piercing with needlelike stylets and sucking through a food channel (Figure 20-18B). In honey bees the labium forms a flexible and contractile “tongue” covered with many hairs. When a bee plunges its proboscis into nectar, the tip of the tongue bends upward and moves back and forth rapidly. Liquid enters the tube by capillarity and is drawn inside continuously by a pumping pharynx. In butterflies and moths, mandibles are usually absent, and the maxillae form a long sucking proboscis (Figure 20-18C) for drawing nectar from flowers. At rest the proboscis coils into a flat spiral. In feeding it extends, and fluid is pumped inside by pharyngeal muscles.
House flies, blow flies, and fruit flies have sponging and lapping mouthparts (Figure 20-18D). At the apex of the labium is a pair of large, soft lobes with grooves on the lower surface that serve as food channels. These flies lap up liquid food or liquefy food first with salivary secretions. Horse flies not only sponge up surface liquids but bite into skin with slender, tapering mandibles and then sponge up blood.
Biting mouthparts such as those of grasshoppers and many other herbivorous insects are adapted for seizing and crushing food (Figure 20-18A); those of most carnivorous insects are sharp and pointed for piercing their prey. Mandibles of chewing insects are strong, toothed plates whose edges can bite or tear while the maxillae hold the food and pass it toward the mouth. Enzymes secreted by the salivary glands add chemical action to the chewing process.
Circulation
A tubular heart in the pericardial cavity (Figure 20-13) moves hemolymph (blood) forward through the only blood vessel, a dorsal aorta. The heartbeat is a peristaltic wave. Accessory pulsatory organs help move hemolymph into the wings and legs, and flow is also facilitated by various body movements. Hemolymph consists of plasma and amebocytes and apparently has little to do with oxygen transport.
Gas Exchange
Terrestrial animals require efficient respiratory systems that permit rapid oxygen and carbon dioxide exchange but at the same time restrict water loss. In insects this is the function of the tracheal system, an extensive network of thin-walled tubes that branch into every part of the body (Figure 20-19). The tracheal trunks open to the outside by paired spiracles, usually two on the thorax and seven or eight on the abdomen. A spiracle may be merely a hole in the integument, as in primary wingless insects, but there is usually a valve or some sort of closing mechanism that reduces water loss. Evolution of such a device must have been very important in enabling insects to move into drier habitats. A spiracle may also possess a filtering device such as a sieve plate or a set of interlocking bristles that may prevent entrance of water, parasites, or dust into the tracheae.
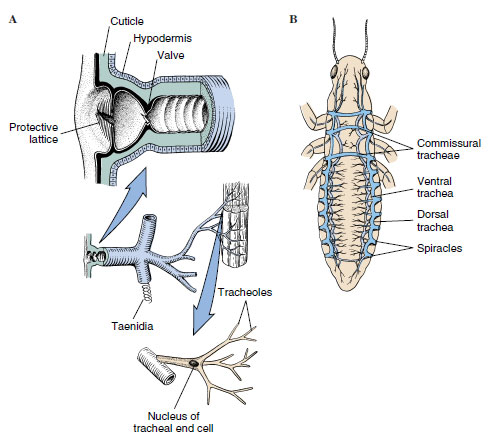 |
| Figure 20-19 A, Relationship of spiracle, tracheae, taenidia (chitinous bands that strengthen the tracheae), and tracheoles (diagrammatic). B, Generalized arrangement of insect tracheal system (diagrammatic). Air sacs and tracheoles not shown. |
Tracheae are composed of a single layer of cells and are lined with cuticle that is shed along with the outer cuticle, during molts. Spiral thickenings of the cuticle (called taenidia) support the tracheae and prevent their collapse. Tracheae branch out into smaller tubes, ending in very fine, fluid-filled tubules called tracheoles (lined with cuticle, but not shed at ecdysis), which branch into a fine network over the cells. In large insects the largest tracheae may be several millimeters in diameter but taper down to 1 to 2 µm. Tracheoles then taper to 0.5 to 0.1 µm in diameter. In one stage of silkworm larvae it is estimated that there are 1.5 million tracheoles! Scarcely any living cell is more than a few micrometers away from a tracheole. In fact, the ends of some tracheoles actually indent the membranes of the cells they supply, so that they terminate close to mitochondria. The tracheal system affords efficient transport without use of oxygen-carrying pigments in hemolymph.
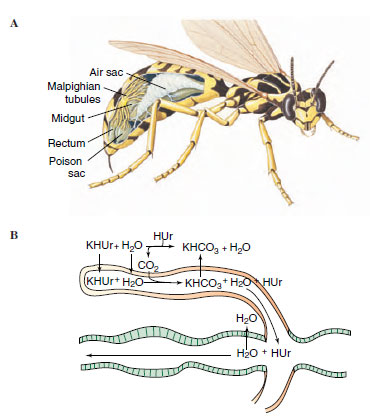 |
| Figure 20-20 Malpighian tubules of insect. A, Malpighian tubules are located at the juncture of the midgut and hindgut (rectum) as shown in the cutaway view of a wasp. B, Function of malpighian tubules. Solutes, especially potassium, are actively secreted into upper tubules. Water and potassium acid urate (KHUr) follow. Potassium is resorbed in the lower tubules, and water and other solutes are resorbed in the rectum. |
The tracheal system may also include air sacs, which are apparently dilated tracheae without taenidia (Figure 20-20A). They are thin walled and flexible and are mostly in the body cavity but also in appendages. In many insects the air sacs increase the volume of air inspired and expired. Muscular movements in the abdomen draw air into the tracheae and expand the sacs, which collapse on expiration. In some insects— locusts, for example—additional pumping is provided by telescoping the abdomen, pumping with the prothorax, or thrusting the head forward and backward. In some insects, air sacs have functions other than respiratory. For example, they may allow internal organs to change in volume during growth without changing the shape of the insect, and they reduce the weight of large insects. In some very small insects, gas transport occurs entirely by diffusion along a concentration gradient. Consumption of oxygen causes a reduced pressure in the tracheae that sucks air in through the spiracles.
The tracheal system is an adaptation for air breathing, but many insects (nymphs, larvae, and adults) live in water. In small, soft-bodied aquatic nymphs, gaseous exchange may occur by diffusion through the body wall, usually into and out of a tracheal network just under the integument. Aquatic nymphs of stoneflies and mayflies have tracheal gills, which are thin extensions of the body wall containing a rich tracheal supply. Gills of dragonfly nymphs are ridges in the rectum (rectal gills) where gas exchange occurs as water enters and leaves.
Excretion and Water Balance
Insects and spiders have a unique excretory system consisting of malpighian tubules that operate in conjunction with specialized glands in the wall of the rectum. Malpighian tubules, variable in number, are thin, elastic, blind tubules attached to the juncture between the midgut and hindgut (Figures 20-13 and 20-20A). Free ends of the tubules lie in the hemocoel and are bathed in hemolymph.
The mechanism of urine formation in malpighian tubules of herbivorous insects appears to depend on active secretion of potassium into the tubules (Figure 20-20B). This primary secretion of ions pulls water along with it by osmosis to produce a potassium-rich fluid. Other solutes and waste materials also are secreted or diffuse into the tubule. The predominant waste product of nitrogen metabolism in most insects is uric acid, which is virtually insoluble in water. Uric acid enters the upper end of the tubule, where the pH is slightly alkaline, as relatively soluble potassium and urate (abbreviated KHUr in Figure 20-20). As formative urine passes into the lower end of the tubule, potassium combines with carbon dioxide, is reabsorbed as potassium bicarbonate (KHCO3), the pH changes to acidic (pH 6.6), and insoluble uric acid (HUr) precipitates out. As urine drains into the intestine and passes through the hindgut, specialized rectal glands absorb chloride, sodium (and in some cases potassium), and water.
Since water requirements vary among different types of insects, this ability to cycle water and salts is very important. Insects living in dry environments may resorb nearly all water from the rectum, producing a nearly dry mixture of urine and feces. Leaf-feeding insects ingest and excrete quantities of fluid. Freshwater larvae need to excrete water and conserve salts. Insects that feed on dry grains need to conserve water and excrete salt.
Nervous System
The nervous system in general resembles that of larger crustaceans, with a similar tendency toward fusion of ganglia (Figure 20-13). A number of insects have a giant fiber system. There is also a stomodeal nervous system that corresponds in function to the autonomic nervous system of vertebrates. Neurosecretory cells located in various parts of the brain have an endocrine function, but, except for their role in molting and metamorphosis, little is known of their activity.
Sense Organs
Along with neuromuscular coordination, insects have unusually keen sensory perception. Their sense organs are mostly microscopic and are located chiefly in the body wall. Each type usually responds to a specific stimulus. The various organs are receptive to mechanical, auditory, chemical, visual, and other stimuli.
Mechanoreception: Mechanical stimuli, those involving touch, pressure, and vibration, are picked up by sensilla. A sensillum may be simply a seta, or hairlike process, connected with a nerve cell, a nerve ending just under the cuticle and lacking a seta, or a more complex organ (scolopophorous organ) consisting of sensory cells with their endings attached to the body wall. Such organs are widely distributed over the antennae, legs, and body.
Auditory Reception: Very sensitive setae (hair sensilla) or tympanal organs may detect airborne sounds. In tympanal organs a number of sensory cells (ranging from a few to hundreds) extend to a very thin tympanic membrane that encloses an air space in which vibrations can be detected. Tympanal organs occur in certain Orthoptera (Figure 20-4), Homoptera, and Lepidoptera. Some insects are fairly insensitive to airborne sounds but can detect vibrations reaching them through the substrate. Organs on the legs usually detect vibrations of the substrate.
Chemoreception: Chemoreceptors (for taste or smell) are usually bundles of sensory cell processes often located in sensory pits. These are often on mouthparts, but in ants, bees, and wasps they are also on the antennae, and butterflies, moths, and flies also have them on the legs. Chemical sense is generally keen, and some insects can detect certain odors for several kilometers. Many patterns of insect behavior such as feeding, mating, habitat selection, and host-parasite relations are mediated through chemical senses. These senses play a crucial role in responses of insects to artificial repellents and attractants.
Visual Reception: Insect eyes are of two types, simple and compound. Simple eyes are found in some nymphs and larvae and in many adults. Most insects have three ocelli on the head. Honey bees probably use ocelli to monitor light intensity but not to form images.
Most adult insects have compound eyes, which may cover much of the head. They consist of thousands of ommatidia—6300 in the eye of a honey bee, for example. The structure of the compound eye is similar to that of crustaceans (Figure 20-21). An insect such as a honey bee can see simultaneously in almost all directions around its body, but it is more myopic than humans, and images, even of nearby objects, are fuzzy. However, most flying insects rate much higher than humans in flicker-fusion tests. Flickers of light become fused in human eyes at a frequency of 45 to 55 per second, but bees and blow flies can distinguish as many as 200 to 300 separate flashes of light per second. This is probably an advantage in analyzing a fastchanging landscape during flight.
A bee can distinguish colors, but its sensitivity begins in the ultraviolet range, which human eyes cannot see, and extends into the orange; honey bees cannot distinguish shades of red from shades of gray.
Other Senses: Insects also have welldeveloped senses for temperature, especially on the antennae and legs, and for humidity, proprioception (sensation of muscle stretch and body position), gravity, and other physical properties.
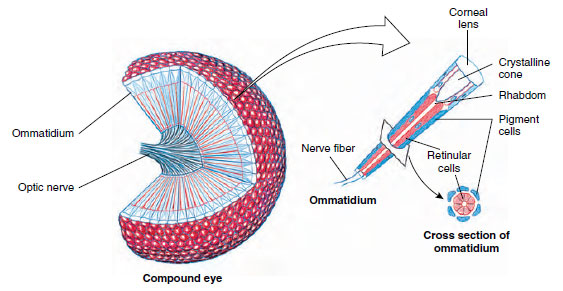 |
| Figure 20-21 Compound eye of an insect. A single ommatidium is shown enlarged to the right. |
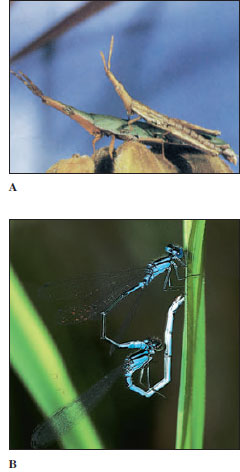 |
| Figure 20-22 Copulation in insects (see also Figure 20-9B). A, Omura congrua (order Orthoptera) are a kind of grasshopper found in Brazil. B, Bluet damselflies Enallagma sp. (order Odonata) are common throughout North America. Here, the male still grasps the female after copulation as the female (white abdomen) lays eggs. |
Neuromuscular Coordination
Insects are active creatures with excellent neuromuscular coordination. Arthropod muscles are typically cross-striated, just as vertebrate skeletal muscles are. A flea can leap a distance of 100 times its own length, and an ant can carry in its jaws a load greater than its own weight. This sounds as though insect muscle were stronger than that of other animals. Actually, however, the force a particular muscle can exert is related directly to its cross-sectional area, not its length. Based on maximum load moved per square centimeter of cross section, the strength of insect muscle is relatively the same as that of vertebrate muscle. The illusion of great strength of insects (and other small animals) is simply a consequence of small body size.
Reproduction
Sexes are separate in insects, and fertilization is usually internal. Parthenogenesis occurs prominently in Homoptera and Hymenoptera. Insects have various means of attracting mates. A female moth releases a powerful pheromone that males can detect for a great distance. Fireflies use flashes of light; some insects find each other by sounds or color signals and by various kinds of courtship behavior.
Males usually deposit sperm in a female’s vagina during copulation (Figures 20-13 and 20-22). In some orders sperm are encased in spermatophores that may be transferred at copulation or deposited on the substratum to be picked up by a female. A male silverfish deposits a spermatophore on the ground, then spins signal threads to guide a female to it. During evolutionary transition of ancestral insects from aquatic to terrestrial life, spermatophores were used widely and copulation evolved much later.
Usually sperm are stored in the spermatheca of a female in numbers sufficient to fertilize more than one batch of eggs. Many insects mate only once during their lifetime, but male damselflies copulate several times per day.
Insects usually lay a great many eggs. A queen honey bee, for example, may lay more than 1 million eggs during her lifetime. On the other hand, some flies are viviparous and bring forth only a single offspring at a time. Insects that make no provision for care of their young may lay many more eggs than do insects that provide for their young or those that have a very short life cycle.
Most species lay their eggs in a particular habitat to which visual, chemical, or other cues guide them. Butterflies and moths lay their eggs on the specific kind of plant on which the caterpillar must feed. A tiger moth may look for a pigweed, a sphinx moth for a tomato or tobacco plant, and a monarch butterfly for a milkweed plant (Figure 20-23). Insects whose immature stages are aquatic characteristically lay their eggs in water (Figure 20-24). A tiny braconid wasp lays her eggs on the caterpillar of the sphinx moth where they will feed and pupate in tiny white cocoons (Figure 20-17). An ichneumon wasp, with unerring accuracy, seeks out a certain kind of larva in which her young will live as parasitoids. Her long ovipositors may have to penetrate 1 to 2 cm of wood to find a larva of a wood wasp or a wood-boring beetle in which she will deposit her eggs (Figure 20-10).
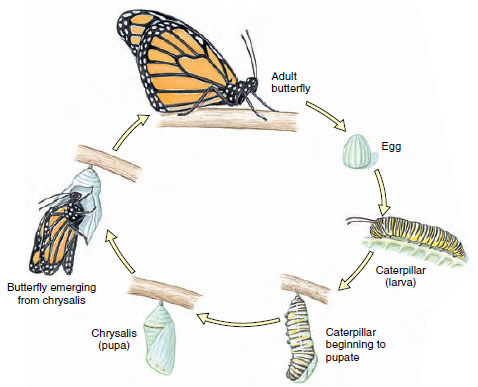 |
 |
| Figure 20-23 Complete (holometabolous) metamorphosis in a butterfly, Danaus plexippus. Eggs hatch to produce first of several larval instars. Last larval instar molts to become a pupa. Adult emerges at pupal molt. |
Figure 20-24 A, Mosquito Culex (order Diptera) lays her eggs in small packets or rafts on the surface of standing or slowly moving water. B, Mosquito larvae are the familiar wrigglers of ponds and ditches. To breathe, they hang head down, with respiratory tubes projecting through the surface film of water. Motion of vibratile tufts of fine hairs on the head brings a constant supply of food. |
Metamorphosis and Growth
Early development occurs within the egg, and hatching young escape from the egg in various ways. During postembryonic development most insects change in form, undergoing metamorphosis (Figure 20-23). During this period they must undergo a series of molts to grow, and each stage between molts is called an instar.
Although metamorphosis occurs in many animals, insects illustrate it more dramatically than any other group. The transformation, for instance, of a hickory horned devil caterpillar into a beautiful royal walnut moth represents an astonishing morphological change. In insects metamorphosis is associated with evolution of wings, which are restricted to the reproductive stage where they are most beneficial.
Holometabolous
Metamorphosis
Approximately 88% of insects undergo a holometabolous (Gr. holo, complete, + metabole, change) metamorphosis, which separates physiological processes of growth (larva) from those of differentiation (pupa) and reproduction (adult) (Figure 20-23). Each stage functions efficiently without competition with the other stages, for larvae often live in entirely different surroundings and eat different foods from adults. The wormlike larvae, which usually have chewing mouthparts, are known as caterpillars, maggots, bagworms, fuzzy worms, or grubs. After a series of instars a larva forms a case or cocoon around itself and becomes a pupa, or chrysalis, a nonfeeding stage in which many insects pass the winter. When the final molt occurs over winter, the full-grown adult emerges, pale and with wings wrinkled. In a short time the wings expand and harden, and the insect is on its way. The stages, then, are egg, larva (several instars), pupa, and adult (Figure 20-23). Adults undergo no further molting.
Hemimetabolous
Metamorphosis
Some insects undergo a hemimetabolous (Gr. hemi, half, + metabole, change), or gradual (incomplete), metamorphosis. These include grasshoppers, cicadas, mantids, and terrestrial bugs, which have terrestrial young, and mayflies, stoneflies, dragonflies, and aquatic bugs that lay their eggs in water and whose young are aquatic. The young are called nymphs, and their wings develop externally as budlike outgrowths in early instars and increase in size as the animal grows by successive molts and becomes a winged adult (Figures 20-25 and 20-26). Aquatic nymphs in some orders have tracheal gills or other modifications for aquatic life (Figure 20-27). The stages are egg, nymph (several instars), and adult (Figure 20-26).
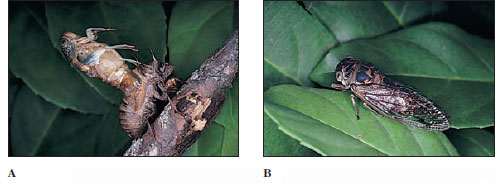 |
| Figure 20-25 A, Ecdysis in a cicada, Tibicen davisi (order Homoptera). The old cuticle splits along a dorsal midline as a result of increased blood pressure and of air forced into the thorax by muscle contraction. The emerging insect is pale, and its new cuticle is soft. The wings will be expanded by blood pumped into veins, and the insect enlarges by taking in air. B, An adult Tibicen davisi. |
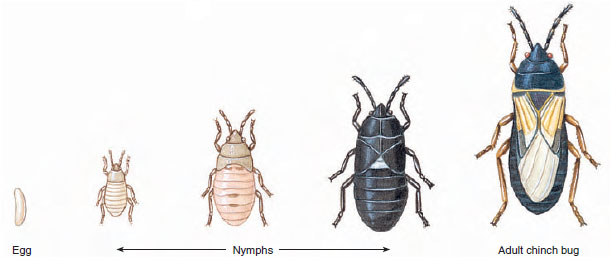 |
| Figure 20-26 Life history of a hemimetabolous insect. |
 |
| Figure 20-27 A, Stonefly, Perla sp. (order Plecoptera). B, Ten-spot dragonfly, Libellula pulchella (order Odonata). C, Nymph (larva) of dragonfly. Both stoneflies and dragonflies have aquatic larvae that undergo gradual metamorphosis. |
Direct Development
A few insects, such as silverfish and springtails, undergo direct development. The young, or juveniles, are similar to adults except in size and sexual maturation. The stages are egg, juvenile, and adult. Such insects include the primitively wingless insects.
Physiology of Metamorphosis
Hormones regulate metamorphosis in insects. Major endocrine organs that are involved in development are the brain, the prothoracic (ecdysial) glands, the corpora cardiaca, and the corpora allata (Figure 36-4).
The intercerebral part of the brain and the ganglia of the nerve cord contain several groups of neurosecretory cells that produce a brain hormone called prothoracicotropic hormone (PTTH). These neurosecretory cells send their axons to paired organs behind the brain, the corpora cardiaca, which serve as storage and release organs for PTTH (and also produce other hormones). PTTH is carried in the hemolymph to the prothoracic gland, a glandular organ that in the head or prothorax produces molting hormone, or ecdysone (ek´duhsone) in response to brain hormone. Ecdysone sets in motion certain processes that lead to casting off of the old cuticle (ecdysis).
Simple molting persists as long as juvenile hormone is present in sufficient amounts, along with molting hormone in the hemolymph, and each molt produces a larger larva. The corpora allata produce juvenile hormone (Figure 36-4).
In later instars, the corpora allata release progressively less juvenile hormone. When juvenile hormone is at a very low level, a larva molts to become a pupa, and cessation of juvenile hormone production in the pupa leads to an adult at the next molt (metamorphosis). Control of development is the same in hemimetabolous insects, except that there is no pupa, and cessation of juvenile hormone production occurs in the final nymphal instar. The corpora allata again become active in adult insects, in which juvenile hormone is important in normal egg production. The prothoracic glands degenerate in adults of most insects, and adults do not molt. Insect hormones have received much interesting experimental study.
For example, if the corpora allata (and thus juvenile hormone) are removed surgically from a larva, the following molt will result in metamorphosis. Conversely, if the corpora allata from a young larva are transplanted into a final larval instar, the latter can be converted into a giant larva, because metamorphosis to the pupa cannot occur.
Diapause
Many animals, including many types of insects, undergo a period of dormancy in their annual life cycle. In temperate zones there may be a period of winter dormancy, called hibernation, or a period of summer dormancy, called estivation, or both. There are periods in the life cycle of many insects when eggs, larvae, pupae, or even adults remain dormant for a long time because external conditions are too harsh or unfavorable for survival in states of normal activity. Thus the life cycle is synchronized with periods of suitable environmental conditions and abundance of food. Most insects enter a dormant state when some factor of the environment, such as temperature, becomes unfavorable, and dormancy continues until conditions again become favorable.
However, some species have a prolonged arrest of growth that occurs regardless of environment, whether or not favorable conditions prevail. This type of dormancy is called diapause (di´a-poz) (Gr. dia, through, dividing into two parts, + pausis, a stopping), and it is an important adaptation to survive adverse environmental conditions. Diapause is genetically determined in each species and sometimes varies between strains within a species, but it is usually initiated by a particular signal. In the environment of an insect, such signals forecast adverse conditions to come, for example, lengthening or shortening of the days. Thus photoperiod, or day length, is often the signal that initiates diapause. After diapause is initiated, another environmental signal is usually required to end it. Such signal may be return of favorable temperature after a prolonged period of cold or an occasion of rain after a dry period, as in a desert.
Diapause always occurs at the end of an active growth stage of the molting cycle so that, when the diapause period is over, the insect is ready for another molt. One species of the ant Myrmica reaches the third instar stage in late summer. Many larvae do not develop beyond this point until the following spring, even if temperatures are mild or if the larvae are kept in a warm laboratory.
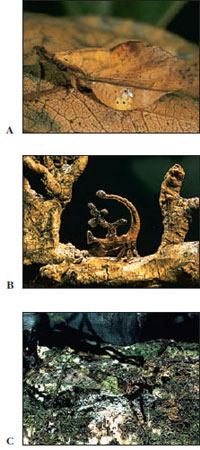 |
| Figure 20-29 Camouflage in insects. A, Estigena pardalis (order Lepidoptera) in Java resembles a dead leaf. B, Bizarre processes from the thorax of a treehopper from Mexico, Sphongophorus sp. (order Homoptera), masquerade as parts of the twig on which it feeds. C, Broken outlines and color of a katydid (Dysonia sp., order Orthoptera) in Costa Rica give it the appearance of the leaves on which it has been feeding. |
Defense
Insects as a group display many colors. This is especially true of butterflies, moths, and beetles. Even within a species the color pattern may vary in a seasonal way, and there also may be color differences between males and females. Some of the color patterns in insects are probably highly adaptive, such as those for protective coloration, warning coloration, and mimicry (Figures 20-28 and 20-29).
Besides color, insects have other methods of protecting themselves. The cuticular exoskeleton affords good protection for many of them. Some, such as stink bugs, have repulsive odors and tastes; other protect themselves by a good offense, for many are very aggressive and fight (for example, bees and ants); and still others are swift in running for cover when danger threatens.
Many insects practice chemical warfare in a variety of ingenious ways. Some repel an assault by virtue of their bad taste, odor, or poisonous properties; others use chemical exudates that mechanically prevent a predator from attacking. Caterpillars of some monarch butterflies (Figure 20-28) assimilate cardiac glycosides from certain species of milkweed (family Asclepiadaceae); this substance confers unpalatability on the butterflies after metamorphosis and induces vomiting in their predators. Bombardier beetles, on the other hand, produce an irritating spray, which they aim accurately at attacking ants or other enemies.
Behavior and Communication
The keen sensory perceptions of insects make them extremely responsive to many stimuli. Stimuli may be internal (physiological) or external (environmental), and responses are governed by both the physiological state of the animal and the pattern of nerve pathways traveled by the impulses. Many responses are simple, such as orientation toward or away from the stimulus, for example, avoidance of light by a cockroach, or attraction of carrion flies to the odor of dead flesh.
Much behavior of insects, however, is not a simple matter of orientation but involves a complex series of responses. A pair of tumble bugs, or dung beetles, chew off a bit of dung, roll it into a ball, and roll the ball laboriously to where they intend to bury it, after laying their eggs in it (Figure 20-30). Cicadas slit the bark of a twig and then lay an egg in each of the slits. Female potter wasps Eumenes scoop up clay into pellets, carry them one by one to a building site, and fashion them into dainty little narrownecked clay pots, into each of which the wasps lay an egg. Then a mother wasp hunts and paralyzes a number of caterpillars, pokes them into the opening of a pot, and closes up the opening with clay. Each egg, in its own protective pot, hatches to find a well-stocked larder of food.
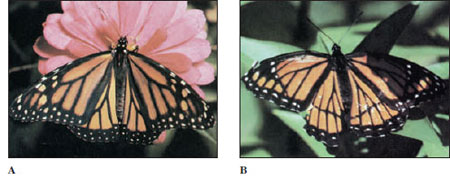 |
 |
| Figure 20-28 Mimicry in butterflies. A, Monarch butterfly is distasteful to, and avoided by, birds because as a caterpillar it fed on the acrid milkweed. B, The monarch is mimicked by the smaller viceroy butterfly, Limenitis archippus, which feeds on willows and is presumably tasteful to birds, but is not eaten because it so closely resembles the monarch in color and markings. This kind of mimicry is called Batesian mimicry. |
Figure 20-30 Tumble bugs, or dung beetles, Canthon pilularis (order Coleoptera), chew off a bit of dung, roll it into a ball, and then roll it to where they will bury it in soil. One beetle pushes while the other pulls. Eggs are laid in the ball, and larvae feed on the dung. Tumble bugs are black, an inch or less in length, and common in pasture fields. |
Much of such behavior is innate; however, a great deal more learning is involved than was once believed. The potter wasp, for example, must learn where she has left her pots if she is to return to fill them with caterpillars one at a time. Social insects, which have been studied extensively, are capable of most of the basic forms of learning used by mammals. An exception is insight learning. Apparently insects, when faced with a new problem, cannot reorganize their memories to construct a new response.
Insects communicate with each other by means of chemical, visual, auditory, and tactile signals. Chemical signals take the form of pheromones, which are substances secreted by one individual that affect the behavior or physiological processes of another individual. Many pheromones have been described. Like hormones, pheromones are effective in minute quantities. Known functions of various pheromones include attraction of the opposite sex, release of certain behavior patterns (for example, aggregation pheromones to enable mass attack of bark beetles on a tree or for overwintering of ladybird beetles), to fend off aggression, to mark trails and territories, and to signal alarms. Social insects, such as bees, ants, wasps, and termites, can recognize a nestmate—or an alien in the nest—by means of identification pheromones. Social parasites escape detection—and certain destruction—by imitating or duplicating pheromones produced by members of their host colony. Pheromones determine caste in termites and to some extent in ants and bees. They are a primary integrating force in populations of social insects.
Many insect pheromones have been isolated and identified. Traps baited with pheromones have been used for many years to monitor insects of economic importance. They can be used to detect the presence of an insect, such as a new arrival from a neighboring area (tracking spread of European gypsy moth in the United States or the presence of European corn ear worms in a field), or to monitor changes in population levels. Use of pheromone traps has become an important tool to detect potential outbreaks, allowing sufficient time to plan remedial action.
Sound production and reception (phonoproduction and phonoreception) in insects have been studied extensively, and although a sense of hearing is not present in all insects, this means of communication is meaningful to those insects that use it. Sounds serve as warning devices, advertisement of territorial claims, or courtship songs. Sounds of crickets and grasshoppers seem to be concerned with courtship and aggression. Male crickets scrape the modified edges of their forewings together to produce their characteristic chirping. The long, drawn-out sound of male cicadas, a call to attract females, is produced by vibrating membranes in a pair of organs on the ventral side of the basal abdominal segment.
 |
| Figure 20-31 Firefly femme fatale, Photuris versicolor, eating a male Photinus tanytoxus, which she attracted with false mating signals. |
There are many forms of tactile communication, such as tapping, stroking, grasping, and antennae touching, which evoke responses varying from recognition to recruitment and alarm. Certain kinds of flies, springtails, and beetles manufacture their own visual signals in the form of bioluminescence. Best known of a luminescent beetles are fireflies, or lightningbugs (which are neither flies nor bugs, but beetles), in which the flash of light helps to locate a prospective mate. Each species has its own characteristic flashing rhythm produced on the ventral side of the last abdominal segments. Females flash an answer to the species-specific pattern to attract males. This interesting “love call” has been adopted by species of Photuris, which prey on the male fireflies of other species they attract (Figure 20-31).
Social Behavior
Insects rank very high in the animal kingdom in their organization of social groups, and cooperation within the more complex groups depends heavily on chemical and tactile communication. Social communities are not all complex, however. Some community groups are temporary and uncoordinated, as are hibernating associations of carpenter bees or feeding gatherings of aphids. Some are coordinated for only brief periods, and some cooperate more fully, such as tent caterpillars Malacosoma, which join in building a home web and feeding net. However, all of these are open communities with limited social behavior.
In the true societies of some orders, such as Hymenoptera (honey bees and ants) and Isoptera (termites), a complex social life is necessary for perpetuation of the species. They involve all stages of the life cycle, communities are usually permanent, all activities are collective, and there is reciprocal communication and division of labor. The society usually demonstrates polymorphism, or caste differentiation.
|
|
|
Honey bees have one of the most complex organizations in the insect world. Instead of lasting one season, their organization continues for an indefinite period. As many as 60,000 to 70,000 honey bees may live in a single hive. Of these, there are three castes: a single sexually mature female, or queen; a few hundred drones, which are sexually mature males; and the rest are workers, which are sexually inactive genetic females (Figure 20-32).
Workers take care of the young, secrete wax with which they build the six-sided cells of the honeycomb, gather nectar from flowers, manufacture honey, collect pollen, and ventilate and guard the hive. One drone, sometimes more, fertilizes the queen during the mating flight, at which time enough sperm is stored in her spermatheca to last her a lifetime.
Castes are determined partly by fertilization and partly by what is fed to larvae. Drones develop parthenogenetically from unfertilized eggs (and consequently are haploid); queens and workers develop from fertilized eggs (and thus are diploid; see haplodiploidy). Female larvae that will become queens are fed royal jelly, a secretion from the salivary glands of nurse workers. Royal jelly differs from the “worker jelly” fed to ordinary larvae, but components in it that are essential for queen determination have not yet been identified. Honey and pollen are added to worker diet about the third day of larval life. Pheromones in “queen substance,” which is produced by the queen’s mandibular glands, prevent female workers from maturing sexually. Workers produce royal jelly only when the level of “queen substance” pheromone in the colony drops. This change occurs when the queen becomes too old, dies, or is removed. Then workers’ ovaries develop, and they start enlarging a larval cell and feeding a larva royal jelly to produce a new queen. Honey bees have evolved an efficient system of communication by which, through certain body movements, their scouts inform workers of the location and quantity of food sources (Figure 38-23)
Termite colonies contain several castes, consisting of fertile individuals, both males and females, and sterile individuals (Figure 20-33). Some fertile individuals may have wings and may leave the colony, mate, lose their wings, and as king and queen start a new colony. Wingless fertile individuals may under certain conditions substitute for the king or queen. Sterile members are wingless and become workers and soldiers. Soldiers have large heads and mandibles and serve for defense of the colony. As in bees and ants, extrinsic factors cause caste differentiation. Reproductive individuals and soldiers secrete inhibiting pheromones that pass throughout the colony to nymphs through a mutual feeding process, called trophallaxis, so that they become sterile workers. Workers also produce pheromones, and if the level of “worker substance” or “soldier substance” falls, as might happen after an attack by marauding predators, for example, the next generation produces compensating proportions of the appropriate caste.
Ants also have highly organized societies. Superficially, they resemble termites, but they are quite different (belong to a different order) and can be distinguished easily. In contrast to termites, ants are usually dark in color, are hard bodied, and have a constriction posterior to their first abdominal somite.
In ant colonies males die soon after mating and the queen either starts her own new colony or joins some established colony and does the egg laying. Sterile females are wingless workers and soldiers that do the work of the colony: gather food, care for the young, and protect the colony. In many larger colonies there may be two or three types of individuals within each caste.
Ants have evolved some striking patterns of “economic” behavior, such as making slaves, farming fungi, herding “ant cows” (aphids or other homopterans) (Figure 20-34A), sewing their nests together with silk (Figure 20-34B), and using tools.