Nuclear Receptors
Nuclear Receptors
Unlike peptide hormones and epinephrine, which are much too large to pass through cell membranes, steroid hormones (for example, estrogen, testosterone, and aldosterone), are lipid-soluble molecules that readily diffuse through cell membranes. Once inside the cytoplasm, steroid hormones bind selectively to receptor molecules of target cells. While these receptor molecules may be located in either cytoplasm or nucleus, their ultimate site of activity is the nucleus. The hormone-receptor complex, now known as a gene regulatory protein, then activates or inhibits specific genes. As a result, gene transcription is altered, since messenger RNA molecules are synthesized on specific sequences of DNA. Stimulation or inhibition of mRNA formation modifies production of key enzymes, thus setting in motion the hormone’s observed effect (Figure 36-2). Thyroid hormones and the insect-molting hormone, ecdysone, also act through nuclear receptors.
Compared with peptide hormones that act indirectly through second messenger systems, steroids have a direct effect on protein synthesis because they bind a nuclear receptor that modifies specific gene activity.
Control of Secretion Rates of Hormones
Hormones influence cellular functions by altering rates of many different biochemical processes. Many affect enzymatic activity and thus alter cellular metabolism, some change membrane permeability, some regulate synthesis of cellular proteins, and some stimulate release of hormones from other endocrine glands. Because these are all dynamic processes that must adapt to changing metabolic demands, they must be regulated, not merely activated, by the appropriate hormones. This regulation is achieved by precisely controlled release of a hormone into the blood. However, the concentration of a hormone in the plasma depends on two factors: its rate of secretion and the rate at which it is inactivated and removed from the circulation. Consequently, if secretion is to be correctly controlled, an endocrine gland requires information about the level of its own hormone(s) in the plasma.
Many hormones are controlled by negative feedback systems that operate between glands secreting the hormones and target cells (Figure 36-3). A feedback pattern is one in which output is constantly compared with a set point, like a thermostat. For example, CRH (corticotropin-releasing hormone), secreted by the hypothalamus, stimulates the pituitary (the target cells) to release ACTH. ACTH stimulates the adrenal gland (the target cells) to secrete cortisol. As the level of ACTH rises in the plasma, it acts on, or “feeds back” on, the hypothalamus to inhibit release of CRH. Similarly, as cortisol levels rise in the plasma, it “feeds back” on the hypothalamus and pituitary to inhibit release of both CRH and ACTH, respectively. Thus any deviation from the set point (a specific plasma level of each hormone) leads to corrective action in the opposite direction (Figure 36-3). Such a negative feedback system is highly effective in preventing extreme oscillations in hormonal output. However, hormonal feedback systems are more complex than a rigid “closed-loop” system such as the thermostat that controls the central heating system in a house, because hormonal feedback may be altered by input from the nervous system or by metabolites or other hormones.
Extreme oscillations in hormone output do sometimes occur under natural conditions. However, because they have the potential to disrupt finely tuned homeostatic mechanisms, such extreme oscillations, as a result of positive feedback, are highly regulated and possess an obvious shutoff mechanism. For example, hormones controlling parturition (childbirth) are shut off by birth of the young from the uterus; hormones controlling ovulation are shut off by release of an ovum from a follicle.
Unlike peptide hormones and epinephrine, which are much too large to pass through cell membranes, steroid hormones (for example, estrogen, testosterone, and aldosterone), are lipid-soluble molecules that readily diffuse through cell membranes. Once inside the cytoplasm, steroid hormones bind selectively to receptor molecules of target cells. While these receptor molecules may be located in either cytoplasm or nucleus, their ultimate site of activity is the nucleus. The hormone-receptor complex, now known as a gene regulatory protein, then activates or inhibits specific genes. As a result, gene transcription is altered, since messenger RNA molecules are synthesized on specific sequences of DNA. Stimulation or inhibition of mRNA formation modifies production of key enzymes, thus setting in motion the hormone’s observed effect (Figure 36-2). Thyroid hormones and the insect-molting hormone, ecdysone, also act through nuclear receptors.
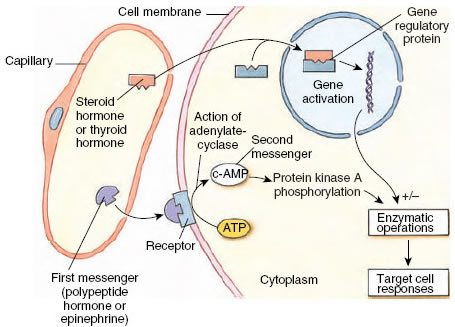 |
Figure 36-2 Mechanisms of hormone action. Peptide hormones and epinephrine act through second messenger systems, as for example, cyclic AMP, shown here. The combination of hormone with a membrane receptor stimulates the enzyme adenylate cyclase to catalyze formation of cyclic AMP (second messenger). Steroid hormones and thyroid hormones penetrate the cell membrane to combine with cytoplasmic or nuclear receptors that alter gene transcription. |
Compared with peptide hormones that act indirectly through second messenger systems, steroids have a direct effect on protein synthesis because they bind a nuclear receptor that modifies specific gene activity.
Control of Secretion Rates of Hormones
Hormones influence cellular functions by altering rates of many different biochemical processes. Many affect enzymatic activity and thus alter cellular metabolism, some change membrane permeability, some regulate synthesis of cellular proteins, and some stimulate release of hormones from other endocrine glands. Because these are all dynamic processes that must adapt to changing metabolic demands, they must be regulated, not merely activated, by the appropriate hormones. This regulation is achieved by precisely controlled release of a hormone into the blood. However, the concentration of a hormone in the plasma depends on two factors: its rate of secretion and the rate at which it is inactivated and removed from the circulation. Consequently, if secretion is to be correctly controlled, an endocrine gland requires information about the level of its own hormone(s) in the plasma.
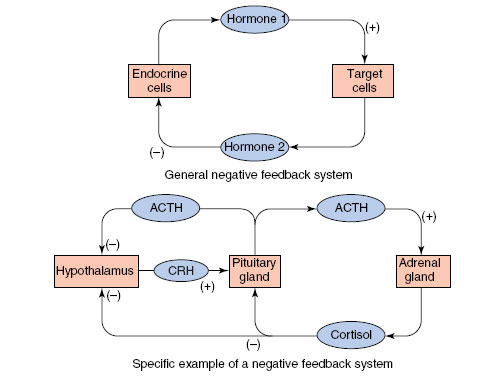 |
| Figure 36-3 Negative feedback systems. |
Many hormones are controlled by negative feedback systems that operate between glands secreting the hormones and target cells (Figure 36-3). A feedback pattern is one in which output is constantly compared with a set point, like a thermostat. For example, CRH (corticotropin-releasing hormone), secreted by the hypothalamus, stimulates the pituitary (the target cells) to release ACTH. ACTH stimulates the adrenal gland (the target cells) to secrete cortisol. As the level of ACTH rises in the plasma, it acts on, or “feeds back” on, the hypothalamus to inhibit release of CRH. Similarly, as cortisol levels rise in the plasma, it “feeds back” on the hypothalamus and pituitary to inhibit release of both CRH and ACTH, respectively. Thus any deviation from the set point (a specific plasma level of each hormone) leads to corrective action in the opposite direction (Figure 36-3). Such a negative feedback system is highly effective in preventing extreme oscillations in hormonal output. However, hormonal feedback systems are more complex than a rigid “closed-loop” system such as the thermostat that controls the central heating system in a house, because hormonal feedback may be altered by input from the nervous system or by metabolites or other hormones.
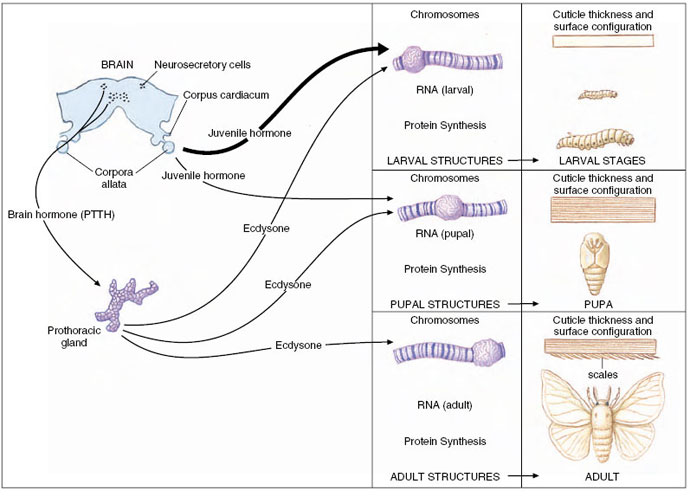 |
| Figure 36-4 Endocrine control of molting in a moth, typical of insects having complete metamorphosis. Many moths mate in spring or summer, and eggs soon hatch into the first of several larval stages, called instars. After the final larval molt, the last and largest larva (caterpillar) spins a cocoon in which it pupates. The pupa overwinters, and an adult emerges in the spring to start a new generation. Juvenile hormone and ecdysone interact to control molting and pupation. Many genes are activated during metamorphosis, as seen by puffing of chromosomes (center column). Puffs form in sequence during successive molts. Changes in cuticle thickness and surface characteristics are shown at right. |
Extreme oscillations in hormone output do sometimes occur under natural conditions. However, because they have the potential to disrupt finely tuned homeostatic mechanisms, such extreme oscillations, as a result of positive feedback, are highly regulated and possess an obvious shutoff mechanism. For example, hormones controlling parturition (childbirth) are shut off by birth of the young from the uterus; hormones controlling ovulation are shut off by release of an ovum from a follicle.




