Protein structure
Primary structure of proteins
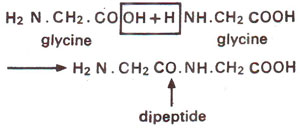
Proteins are polymers of amino acids and at primary level these can be considered to be polypeptide chains in the same manner, as I he nucleic acids are known to be polynucleotides. Isolated amino acids, each consists of a central carbon atom-called alpha carbon—bound to the amino group (NH2), a carboxyl group and a side chain. The differences in amino acids are mainly due to side chains-their shape, size and polarity. The polymer (polypeptide) in a protein consists of amino acids linked with the help of peptide (-CO-NH) bonds. The peptide bond consists of a covalent bond between carboxyl group of one amino acid and amino group of another, shown in Figure 31.1. The protein backbone consists of the repeating series of alpha carbons, carboxyl carbons and amino nitrogen.
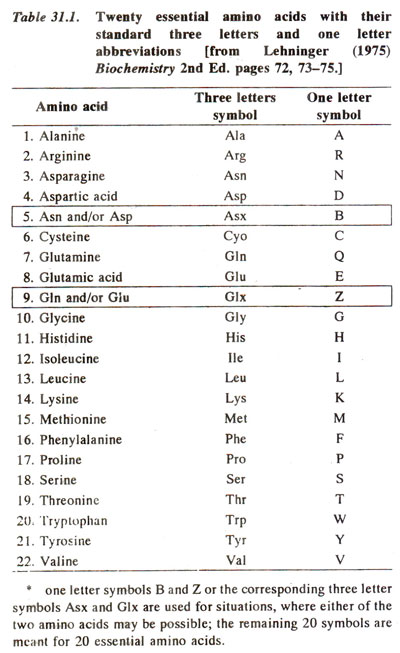
* one letter symbols Band Z or the corresponding three letter symbols Asx and Glx are used for situations, where either of the two amino acids may be possible; the remaining 20 symbols are meant for 20 essential amino acids.
A polypeptide chain always has a spatial (in space) organization. The configuration of backbone of polypeptide chain would mean the secondary structure of protein, of which a particular polypeptide may be a part. These secondary structures fall into three main categories : (i) helices (mainly the alpha helix), (ii) beta strands (backbone is extended or stretched out) or beta sheets (where two or more beta strands are arranged in rows) and (iii) turns connecting the helices and strands (beta turns and omega turns). These three secondary elements are shown in Figure 31.2. The secondary structures are often stabilized by intramolecular hydrogen bonding. These secondary elements can also combine with one another to form motifs, or supersecondary structures, which assemble in the form of a tertiary structure.
Tertiary structure of proteins and 'second half of the genetic code'
Tertiary structure refers to three dimensional structure of a protein. While the secondary structure is obtained by folding and intramolecular bonding in polypeptide, tertiary structure is stabilized by side chains of amino acids. This three-dimensional structure is important, since it forms sites of specific activity, particularly in case of enzymes. An enzyme would lose its specific activity only through alterations in tertiary structure of protein by whatever factors it is brought about.
It has been shown repeatedly that the primary structure of a protein (or in other words the amino acid sequence) is sufficient to specify the tertiary structure of a molecule. However, it is not yet possible to predict the tertiary structure of a protein from its amino acid sequence, because the rules which govern the transformation of a particular amino acid sequence into a specific tertiary structure are not fully understood so far. Hundreds of laboratories around the world are busy working on this problem, which is often described as protein folding problem or also as Second Half of the Genetic Code.
Primary, secondary and tertiary structures have been discussed with respect to single polypeptide units. However, in a particular protein, more than one polypeptides may be associated to give rise to a complex macromolecule, which is biologically a functional unit. This structure obtained through combination of more than one polypeptides would be quaternary structure. Polypeptide units of this quaternary structure would have their own primary, secondary and tertiary structures. It is important to know that, while the mechanism of synthesis of polypeptides (primary structure) is now known in considerable detail, little is known about how particular secondary, tertiary and quaternary structures can be achieved by polypeptides.